Health
THE WPATH TAPES: Behind-The-Scenes Recordings Reveal What Top Gender Doctors Really Think About Sex Change Procedures

 From the Daily Caller News Foundation
From the Daily Caller News Foundation
By MEGAN BROCK AND KATE ANDERSON
The World Professional Association for Transgender Health (WPATH) is the leading authority in the field of gender medicine. Its guidance is routinely used by top medical associations in the U.S. and abroad, while its standards of care inform insurance companies’ approach to coverage policies.
But behind closed doors, top WPATH doctors discussed, and at times seemed to challenge, the organization’s own published guidelines for sex change procedures and acknowledged pushing experimental medical interventions that can have devastating and irreversible complications, according to exclusive footage obtained by the Daily Caller News Foundation.
WPATH published highly influential clinical guidance called “Standards of Care for the Health of Transgender and Gender Diverse People, Version 8” (SOC 8), which recommends the use of invasive medical interventions such as puberty blockers, cross-sex hormones and sex change surgeries, calling them “safe and effective.”
The DCNF filed a series of public records requests to WPATH SOC 8 co-authors who are employed at taxpayer-funded institutions, making their emails subject to open records laws. Buried in more than 100 pages of responsive records from the University of Nevada was a series of emails between prominent WPATH members and leaders, including WPATH Global Education Institute (GEI) Co-Chair Gail Knudson, that were sent in 2022. In one email, Knudson sent a colleague the link to a folder containing nearly 30 hours of recordings from WPATH’s GEI summit in September 2022 in Montreal, Canada, which included sessions on mental health, puberty blockers, cross-sex hormones and sex change surgery.
These sessions provided WPATH members with in-depth education on the clinical application of topics addressed in the SOC 8 treatment guidelines. However, the footage reveals WPATH-affiliated doctors advocating for children to undergo risky sex change procedures and even pushing for these treatments for patients struggling with severe mental health issues. Several sessions were dedicated exclusively to treating children and included recommendations for minors to receive puberty blockers, cross-sex hormones and surgeries.
For instance, WPATH guidance recommends addressing a patient’s mental health issues before giving them sex change medical interventions. However, in one recorded session, a WPATH faculty member and gender doctor claimed that mental health issues don’t necessarily affect a patient’s ability to receive cross-sex hormones.
In another video, a doctor told attendees children should be informed that cross-sex hormones will likely make them infertile but admitted that he will prescribe them anyway if a child says they want the treatment, regardless of the future consequences.
A surgeon euphemistically referred to a phalloplasty procedure, a surgical series that includes obliterating the vaginal cavity and creating a fake penis with harvested tissue, as an “adventure” for young people. He did this despite later admitting that those same procedures will “definitely” have “complications,” such as permanent issues with bladder function and tissue death.
One physician called the entire field of cross-sex hormones “off-label,” referring to the concept of drugs being used for alternative purposes than what they were approved for. The doctor went on to say that female patients might actually appreciate drug side effects that cause them to lose hair, because they’d look “more like men.”
The Food and Drug Administration says that when it approves a drug, healthcare providers generally may prescribe that drug for an unapproved use, or off-label, when “they judge that it is medically appropriate for their patient.”
In several other videos, doctors argued in favor of transitioning patients who experience psychotic episodes. One admitted that some of his patients with schizophrenia have to be careful how much cross-sex hormones they take or they can’t “keep the voices down.”
The DCNF consulted medical professionals from respected organizations, such as Do No Harm, who all argued that the comments from WPATH-affiliated doctors show that the transgender medical industry does not have patients’ best interests at heart.
While the average person, nationally and internationally, likely has never heard of WPATH, the modern medical industry is deeply tied to the organization and relies on it to dictate the standards of care for transgender medicine. WPATH’s guidelines are cited as criteria for obtaining insurance coverage by both private insurance companies and tax-funded insurance plans, positioning them as a lynchpin of the sex reassignment industry.
Additionally, their guidelines help inform policy statements from major medical and professional organizations, such as the American Academy of Pediatrics (AAP), the American Psychological Association and the Endocrine Society. The AAP is currently being sued by Isabelle Ayala, a former patient who was medically transitioned as a child, for allegedly rushing her through sex change medical procedures.
There’s been an explosion in the number of young people, including children, being put on hormones and puberty blockers and getting sex change surgeries, according to a study published in August 2023 by the JAMA Network. This surge has been fueled, in part, by groups like Planned Parenthood, which distributes cross-sex hormones to patients as young as 16. Planned Parenthood saw a roughly 125% jump in the number of transgender services it provided between 2020 and 2022.
Twenty-three states, however, have enacted legislation preventing doctors from performing sex change surgeries on minors amid backlash from concerned parents and doctors who don’t subscribe to the WPATH-endorsed “gender-affirming care” model. Gender-affirming care is another euphemism used by medical professionals to describe the idea that doctors should affirm a patient’s wish to live as the opposite biological sex through social transitioning, hormone therapy and even surgery.
The SOC 8 was released just days ahead of the 2022 symposium and contained several significant changes to how doctors and medical institutions implemented transgender medical treatment. For instance, WPATH removed minimum age requirements criteria that established when a child can or should receive transgender medical services such as puberty blockers, cross-sex hormones, and sex reassignment surgeries.
WPATH’s previous guidelines recommended that hormone therapy be given once a patient was over the age of 16, but the updated version removed this barrier and suggests hormone therapy begin at the first signs of sexual maturity.
The videos obtained by the DCNF give the first glimpse at how doctors and mental health professionals discussed implementing the new guidelines. To highlight the most significant portions of the content obtained in the records requests, the DCNF has decided to publish a series of articles collectively called “The WPATH Tapes.”
Following this release, the DCNF intends to publish all of the videos in their entirety in order to provide the public with necessary information about WPATH’s approach to medical care and shine a light on an influential organization that has largely remained anonymous until now.
The WPATH Tapes Table of Contents:
- Video Shows Prominent Doctors Acknowledging, And Even Challenging, The Experimental Nature Of Sex Change Drugs
- Top Psychiatrist Argues Schizophrenic Patients Can Consent To Sex Change Surgeries
- ‘Keep The Voices Down’: In Unearthed Video, Doctors Discuss Putting Mentally Ill Patients, Including Kids, On Hormones
- Gender Doctor Calls Genital Surgery An ‘Adventure’ For Young People While Describing Grisly Complications
- ‘No Idea About Their Fertility’: Gender Doctors Shed Light On Grim Reality Facing Kids Considering Sex Changes
- Leader Of Gender Medicine Org Says Binary Sex ‘Doesn’t Really Hold True,’ Cheers On ‘Deconstructed’ Biology
- Private Footage Reveals Leading Medical Org’s Efforts To ‘Normalize’ Gender Ideology
Business
Canada has fewer doctors, hospital beds, MRI machines—and longer wait times—than most other countries with universal health care
From the Fraser Institute
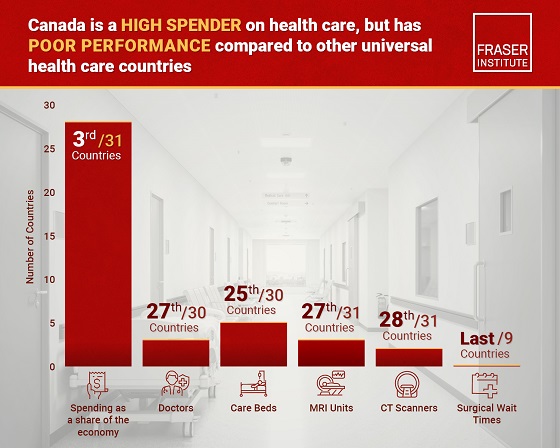
Despite a relatively high level of spending, Canada has significantly fewer doctors, hospital beds, MRI machines and CT scanners compared to other countries with universal health care, finds a new study released today by the Fraser Institute, an independent, non-partisan Canadian public policy think-tank.
“There’s a clear imbalance between the high cost of Canada’s health-care system and the actual care Canadians receive in return,” said Mackenzie Moir, senior policy
analyst at the Fraser Institute and author of Comparing Performance of Universal Health-Care Countries, 2025.
In 2023, the latest year of available comparable data, Canada spent more on health care (as a percentage of the economy/GDP, after adjusting for population age) than
most other high-income countries with universal health care (ranking 3rd out of 31 countries, which include the United Kingdom, Australia and the Netherlands).
And yet, Canada ranked 27th (of 30 countries) for the availability of doctors and 25th (of 30) for the availability of hospital beds.
In 2022, the latest year of diagnostic technology data, Canada ranked 27th (of 31 countries) for the availability of MRI machines and 28th (of 31) for CT scanners.
And in 2023, among the nine countries with universal health-care systems included in the Commonwealth Fund’s International Health Policy Survey, Canada ranked last for the percentage of patients able to make same- or next-day appointments when sick (22 per cent) and had the highest percentage of patients (58 per cent) who waited two months or more for non-emergency surgery. For comparison, the Netherlands had much higher rates of same- or next-day appointments (47 per cent) and much lower waits of two months or more for non-emergency surgery (20 per cent).
“To improve health care for Canadians, our policymakers should learn from other countries around the world with higher-performing universal health-care systems,”
said Nadeem Esmail, director of health policy at the Fraser Institute.
Comparing Performance of Universal Health Care Countries, 2025
- Of the 31 high-income universal health-care countries, Canada ranks among the highest spenders, but ranks poorly on both the availability of most resources and access to services.
- After adjustments for differences in the age of the population of these 31 countries, Canada ranked third highest for spending as a percentage of GDP in 2023 (the most recent year of comparable data).
- Across 13 indictors measured, the availability of medical resources and timely access to medical services in Canada was generally below that of the average OECD country.
- In 2023, Canada ranked 27th (of 30) for the relative availability of doctors and 25th (of 30) for hospital beds dedicated to physical care. In 2022, Canada ranked 27th (of 31) for the relative availability of Magnetic Resonance Im-aging (MRI) machines, and 28th (of 31) for CT scanners.
- Canada ranked last (or close to last) on three of four indicators of timeliness of care.
- Notably, among the nine countries for which comparable wait times measures are available, Canada ranked last for the percentage of patients reporting they were able to make a same- or next-day appointment when sick (22%).
- Canada also ranked eighth worst for the percentage of patients who waited more than one month to see a specialist (65%), and reported the highest percentage of patients (58%) who waited two months or more for non-emergency surgery.
- Clearly, there is an imbalance between what Canadians get in exchange for the money they spend on their health-care system.
Mackenzie Moir
Senior Policy Analyst, Fraser Institute
Health
New report warns WHO health rules erode Canada’s democracy and Charter rights

The Justice Centre for Constitutional Freedoms has released a new report titled Canada’s Surrender of Sovereignty: New WHO health regulations undermine Canadian democracy and Charter freedoms. Authored by Nigel Hannaford, a veteran journalist and researcher, the report warns that Canada’s acceptance of the World Health Organization’s (WHO) revised International Health Regulations (IHR) represents a serious erosion of national independence and democratic accountability.
The IHR amendments, which took effect on September 19, 2025, authorize the WHO Director-General to declare global “health emergencies” that could require Canada to follow directives from bureaucrats in Geneva, bypassing the House of Commons and the will of Canadian voters.
The WHO regards these regulations as “binding,” despite having no ability or legal authority to impose such regulations. Even so, Canada is opting to accept the regulations as binding.
By accepting the WHO’s revised IHR, the report explains, Canada has relinquished its own control over future health crises and instead has agreed to let the WHO determine when a “pandemic emergency” exists and what Canada must do to respond to it, after which Canada must report back to the WHO.
In fact, under these International Health Regulations, the WHO could demand countries like Canada impose stringent freedom-violating health policies, such as lockdowns, vaccine mandates, or travel restrictions without debate, evidence review, or public accountability, the report explains.
Once the WHO declares a “Pandemic Emergency,” member states are obligated to implement such emergency measures “without delay” for a minimum of three months.
Importantly, following these WHO directives would undermine government accountability as politicians may hide behind international “commitments” to justify their actions as “simply following international rules,” the report warns.
Canada should instead withdraw from the revised IHR, following the example of countries like Germany, Austria, Italy, Czech Republic, and the United States. The report recommends continued international cooperation without surrendering control over domestic health policies.
Constitutional lawyer Allison Pejovic said, “[b]y treating WHO edicts as binding, the federal government has effectively placed Canadian sovereignty on loan to an unelected international body.”
“Such directives, if enforced, would likely violate Canadians’ Charter rights and freedoms,” she added.
Mr. Hannaford agreed, saying, “Canada’s health policies must be made in Canada. No free and democratic nation should outsource its emergency powers to unelected bureaucrats in Geneva.”
The Justice Centre urges Canadians to contact their Members of Parliament and demand they support withdrawing from the revised IHR to restore Canadian sovereignty and reject blind compliance with WHO directives.
-

 Alberta2 days ago
Alberta2 days agoClick here to help choose Alberta’s new licence plate design
-

 National2 days ago
National2 days agoDemocracy Watch Renews Push for Independent Prosecutor in SNC-Lavalin Case
-

 Business2 days ago
Business2 days agoOver two thirds of Canadians say Ottawa should reduce size of federal bureaucracy
-

 Alberta1 day ago
Alberta1 day agoBusting five myths about the Alberta oil sands
-

 Frontier Centre for Public Policy1 day ago
Frontier Centre for Public Policy1 day agoOttawa Should Think Twice Before Taxing Churches
-

 City of Red Deer1 day ago
City of Red Deer1 day agoPlan Ahead: Voting May Take a Little Longer This Election Day
-

 Energy1 day ago
Energy1 day agoMinus Forty and the Myth of Easy Energy
-

 Health24 hours ago
Health24 hours agoNew report warns WHO health rules erode Canada’s democracy and Charter rights





