Business
US lawmakers accuse Pfizer, Eli Lilly of testing new drugs on prisoners in Communist China

From LifeSiteNews
Two Republicans and two Democrats in the House of Representatives have leveled stunning allegations against two pharmaceutical companies, calling on the U.S. Food and Drug Administration to investigate potential testing of drugs on prisoners of Communist China.
A bipartisan group of Congress members has leveled stunning allegations against pharmaceutical companies Pfizer and Eli Lilly, calling on the U.S. Food & Drug Administration (FDA) to investigate the potential testing of new drugs on prisoners of Communist China.
The letter was sent August 19 to FDA Commissioner Dr. Robert Calf and signed by Select Committee on the Chinese Communist Party (CCP) Chair Rep. John Moolenaar, a Republican from Florida and ranking member and Illinois Democratic Rep. Raja Krishnamoorthi, Health Energy & Commerce Subcommittee ranking member and California Democratic Rep. Anna Eshoo, and Florida Republican Rep. Neal Dunn.
“For over a decade, it appears that U.S. biopharmaceutical companies conducted clinical trials with China’s military organizations, and specifically with medical centers and hospitals affiliated with the People’s Liberation Army’s (PLA), to determine the safety and effectiveness of new drug candidates prior to approval,” the letter reads. “ … we are also concerned that U.S. biopharmaceutical companies have conducted clinical trials with hospital infrastructure located in the Xinjiang Uyghur Autonomous Region (XUAR), where the Chinese Communist Party (CCP) is engaged in genocide of the Uyghur population.”
The lawmakers’ review of publicly available data found that over the last decade major American Pharma companies have conducted “hundreds of clinical trials in China that included at least one entity with PLA in the name as a research trial partner.”
“Even today, one major U.S. biopharmaceutical entity is actively recruiting patients for an advanced Alzheimer drug trial and is partnered with the PLA’s General Hospital and Medical School … and the PLA’s Air Force Medical University. … Previously, another U.S. biopharmaceutical entity used the 307 Hospital of the PLA (307 医院) as the setting for a cancer therapeutic clinical trial.”
Such work not only carries risks of sensitive technology falling into the CCP’s hands, “there are also U.S. biopharmaceutical trials listed on clinicaltrials.gov that were conducted with hospitals located in the XUAR, where credible investigative reports have shown that ethnic minorities in the region are repeatedly forced by the CCP to surrender their body autonomy. As we know, there is simply no ability for firms to conduct due diligence to ensure that clinical trials done in XUAR are voluntary.”
Axios noted that the trials in question concern Pfizer’s kidney cancer drug axitinib (brand name Inlyta), and Eli Lilly’s Alzheimer’s drug donanemab (brand name Kisunla).
The lawmakers asked the FDA to answer several questions related to its knowledge and oversight of such trials and called on the agency to “take on a greater role in protecting U.S. national security interests. With this data, it is clear that the FDA should play a greater role in analyzing U.S. biopharma entities (sic) clinical trial operations in the PRC.”
Pfizer responded that it “is committed to conducting business in an ethical and responsible manner. This includes respecting internationally recognized human rights throughout our operations,” Straight News reported. Eli Lilly claimed that it is “committed to IP protections, and we conduct robust assessments of our partners to ensure they meet Lilly standards for research and data privacy. Further, we oversee their activities when conducting clinical trials to ensure quality and data integrity.”
The allegations come amid a strained public reputation for Big Pharma given its role in the COVID-19 pandemic response.
A large body of evidence has found that mass restrictions on personal and economic activity undertaken in 2020 and part of 2021 caused far more harm than good in terms of personal freedom and economics as well as public health, particularly through the controversial COVID vaccines rushed through development by Pfizer, Moderna, Johnson & Johnson, and the Trump administration.
Yet, so far Big Pharma has largely escaped accountability thanks to the federal Public Readiness and Emergency Preparedness (PREP) Act of 2005. According to the Congressional Research Service (CRS), the PREP Act empowers the federal government to “limit legal liability for losses relating to the administration of medical countermeasures such as diagnostics, treatments, and vaccines.” Near the beginning of the 2020 COVID-19 outbreak, the Trump administration invoked the Act in declaring the virus a “public health emergency.”
Under this “sweeping” immunity, CRS explained, the federal government, state governments, “manufacturers and distributors of covered countermeasures,” and licensed or otherwise-authorized health professionals distributing those countermeasures are shielded from “all claims of loss” stemming from those countermeasures, with the exception of “death or serious physical injury” brought about through “willful misconduct,” a standard that, among other hurdles, requires the offender to have acted “intentionally to achieve a wrongful purpose.”
A handful of states are currently making efforts to hold Pharma companies accountable despite this hurdle, such as Florida’s ongoing grand jury investigation into the vaccines’ manufacturers, and a Kansas lawsuit accusing Pfizer of misrepresentation for calling the shots “safe and effective.”
2025 Federal Election
Canada drops retaliatory tariffs on automakers, pauses other tariffs

 MxM News
MxM News
Quick Hit:
Canada has announced it will roll back retaliatory tariffs on automakers and pause several other tariff measures aimed at the United States. The move, unveiled by Finance Minister François-Philippe Champagne, is designed to give Canadian manufacturers breathing room to adjust their supply chains and reduce reliance on American imports.
Key Details:
- Canada will suspend 25% tariffs on U.S. vehicles for automakers that maintain production, employment, and investment in Canada.
- A broader six-month pause on tariffs for other U.S. imports is intended to help Canadian sectors transition to domestic sourcing.
- A new loan facility will support large Canadian companies that were financially stable before the tariffs but are now struggling.
Diving Deeper:
Ottawa is shifting its approach to the escalating trade war with Washington, softening its economic blows in a calculated effort to stabilize domestic manufacturing. On Tuesday, Finance Minister François-Philippe Champagne outlined a new set of trade policies that provide conditional relief from retaliatory tariffs that have been in place since March. Automakers, the hardest-hit sector, will now be eligible to import U.S. vehicles duty-free—provided they continue to meet criteria that include ongoing production and investment in Canada.
“From day one, the government has reacted with strength and determination to the unjust tariffs imposed by the United States on Canadian goods,” Champagne stated. “We’re giving Canadian companies and entities more time to adjust their supply chains and become less dependent on U.S. suppliers.”
The tariff battle, which escalated in April with Canada slapping a 25% tax on U.S.-imported vehicles, had caused severe anxiety within Canada’s auto industry. John D’Agnolo, president of Unifor Local 200, which represents Ford employees in Windsor, warned the BBC the situation “has created havoc” and could trigger a recession.
Speculation about a possible Honda factory relocation to the U.S. only added to the unrest. But Ontario Premier Doug Ford and federal officials were quick to tamp down the rumors. Honda Canada affirmed its commitment to Canadian operations, saying its Alliston facility “will operate at full capacity for the foreseeable future.”
Prime Minister Mark Carney reinforced the message that the relief isn’t unconditional. “Our counter-tariffs won’t apply if they (automakers) continue to produce, continue to employ, continue to invest in Canada,” he said during a campaign event. “If they don’t, they will get 25% tariffs on what they are importing into Canada.”
Beyond the auto sector, Champagne introduced a six-month tariff reprieve on other U.S. imports, granting time for industries to explore domestic alternatives. He also rolled out a “Large Enterprise Tariff Loan Facility” to support big businesses that were financially sound prior to the tariff regime but have since been strained.
While Canada has shown willingness to ease its retaliatory measures, there’s no indication yet that the U.S. under President Donald Trump will reciprocate. Nevertheless, Ottawa signaled its openness to further steps to protect Canadian businesses and workers, noting that “additional measures will be brought forward, as needed.”
Business
DOGE Is Ending The ‘Eternal Life’ Of Government


From the Daily Caller News Foundation
By David Bossie
In his 1964 “A Time For Choosing” speech, Ronald Reagan famously said, “a government bureau is the nearest thing to eternal life we’ll ever see on this earth.” And for more than 60 years, President Reagan’s words have proven to be true. However, with the historic re-election of President Donald Trump and the creation of the Department of Government Efficiency (DOGE) under the leadership of Elon Musk, the Gipper’s contention is finally being challenged – and not a moment too soon.
The Trump Administration inherited a horribly bloated federal government in dire need of common sense streamlining from top to bottom. For decades, the executive branch has expanded at an incomprehensible rate and along with it, so has waste, fraud, and abuse. Presidents on both sides of the aisle have made promises to tighten the government’s belt, shrink the bureaucracy, and return power to the people where it belongs. Those efforts for the most part – however well-intentioned – never got off the ground. The reality is that when politicians have been forced to choose between a legislative priority and cutting government spending, cuts are always the first casualty. But currently, with our $36 trillion national debt spiraling out of control, reining in the size and scope of government is no longer a choice, but a necessity.
President Trump is the perfect leader for these trying times. He’s battletested and fears nothing – and no challenge is too large. Whether it’s securing the border, growing the economy, forging peace in Ukraine and the Middle East, or negotiating fair trade deals, this president is on a mission to save America. And if any chief executive is going to have success at deconstructing the administrative state, it’s Trump the steel-spined change agent. The shadowy deep state doesn’t scare him, the biased liberal media can’t intimidate him, and this time there are no phony partisan investigations aiming to sidetrack him. Trump made a promise to bring fiscal responsibility back to governing, and along with Musk and DOGE, they’re finally conducting the “audit with teeth” that the American people have been waiting for, and their hard work is turning out to be infectious.
Dear Readers:
As a nonprofit, we are dependent on the generosity of our readers.
Please consider making a small donation of any amount here. Thank you!
With each passing day, a different member of the cabinet is announcing a new cut, discovering a duplicative program, or updating an antiquated system to steer us away from the fiscal cliff that’s rapidly approaching. When the president also happens to be a highly successful businessman, making the business operate more smoothly and for less money is the name of the game. Trump has brought this mindset to the White House and according to recent polling 77 percent favor a full review of government spending.
President Trump is going back to the basics that have become taboo in Washington, like asking fundamental questions about whether an agency has been successful in its mission or if a program is still necessary. In the case of the Education Department, Trump sees an emergency and is not willing to kick the can down the road any longer. The president believes that education excellence for our children is essential so America can compete for generations to come. Drastic reform is long overdue and that means moving education decisions back to state and local officials – and parents. That’s why President Trump is taking the steps to confront the failed status quo and close the underperforming department so we can turnaround lackluster public schools and low-test scores.
Similarly, with the decision to end USAID and slash foreign aid, Trump and DOGE are simply putting America first. America is handing out billions upon billions in taxpayer dollars around the globe on programs that should be spent on fixing our own domestic problems. The plan to decentralize and modernize the Agriculture Department is another great example of thinking outside the box. The American people understand the rationale that downtown Washington, D.C. is the last place decisions about farming should be made. Relocating the department to various hubs around the heartland is common sense.
Additionally, the announcement that the Department of Health and Human Services will cut 20,000 full-time employees is part of President Trump’s vision to “right-size the federal government and unleash the private sector again” in the words of Treasury Secretary Scott Bessent. And word that the Trump Administration is planning to work with Congress to finally defund National Public Radio and the Public Broadcasting Service is welcome news to millions of Americans who believe sending taxpayer funds to biased news outlets is wrong.
DOGE is also doing courageous work at the Social Security Administration (SSA). The amazing efforts to identify individuals who are either deceased, in the country illegally, or otherwise ineligible will help stave off the program’s insolvency, which experts predict is only ten years away. When a DOGE official disclosed that 40 percent of the calls made to SSA are from would-be fraudsters trying to exploit the system, it’s become all too obvious that new safeguards must be adopted.
When it comes to the question of how much money DOGE will ultimately end up saving taxpayers, in the context of our $36 trillion debt crisis, the more the better. However, the overall change in mindset – forcing government to operate efficiently and responsibly like businesses and families – and passing that mindset onto future administrations is perhaps the most critical shift that can be made. In fact, in an ideal scenario, every state, county, and city would have its very own DOGE operation. We must get serious about cutting government waste now or we’ll go bankrupt. That’s just the reality of the situation and President Trump knows it.
David Bossie is the president of Citizens United and served as a senior adviser to the Trump-Pence 2020 campaign. In 2016, Bossie served as deputy campaign manager for Donald J. Trump for President and deputy executive director for the Trump-Pence Transition Team.
-
 illegal immigration2 days ago
illegal immigration2 days agoDespite court rulings, the Trump Administration shows no interest in helping Abrego Garcia return to the U.S.
-
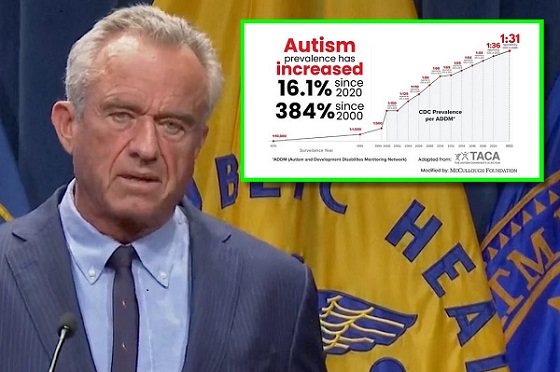
 Autism19 hours ago
Autism19 hours agoRFK Jr. Exposes a Chilling New Autism Reality
-

 2025 Federal Election23 hours ago
2025 Federal Election23 hours agoRCMP Whistleblowers Accuse Members of Mark Carney’s Inner Circle of Security Breaches and Surveillance
-

 2025 Federal Election22 hours ago
2025 Federal Election22 hours agoBureau Exclusive: Chinese Election Interference Network Tied to Senate Breach Investigation
-

 2025 Federal Election2 days ago
2025 Federal Election2 days agoConservative MP Leslyn Lewis warns Canadian voters of Liberal plan to penalize religious charities
-
 International18 hours ago
International18 hours agoUK Supreme Court rules ‘woman’ means biological female
-

 Education2 days ago
Education2 days agoSchools should focus on falling math and reading skills—not environmental activism
-

 Health18 hours ago
Health18 hours agoWHO member states agree on draft of ‘pandemic treaty’ that could be adopted in May








