Health
UK’s NHS set to launch detransitioning services for ‘transgender’ patients

From LifeSiteNews
A report showing that clinical practice for ‘transgenders’ is built on ‘shaky foundations’ prompted the UK’s Health Service to work toward ‘detransitioning’ services.
The National Health Service of England (NHS) is slated to launch its first “detransitioning” service aimed at returning “transgender” individuals to physical conformity with their biological sex.
The move was prompted by the recommendations of a review of Gender Identity Services by pediatrician Dr. Hilary Cass, The Telegraph reported. Dr. Cass’ report found an “exponential” spike in the number of young people who were presented to the UK NHS Gender Identity Service (GIDS) beginning in 2014.
General Practitioners in England were found to be “pressurized to prescribe hormones” by patients who had not consulted with a private clinician, and Dr. Cass concluded that the current practice of so-called “gender medicine” in the U.K., involving the use of puberty blockers and cross-sex hormones, was built on “shaky foundations.”
Dr. Cass reportedly went so far as to recommend that GPs resist efforts by private practitioners to prescribe puberty blockers and cross-sex hormones, “particularly if that private provider is acting outside NHS guidance.”
NHS England has decided to fully adopt Dr. Cass’ recommendations, and on Wednesday published its plans to reform its gender services accordingly. Sir Stephen Prowis, medical director of the NHS, praised Dr. Cass’ work as “invaluable” and said the NHS would now embrace a “fundamentally different and safer model of care for children.”
According to Health Service officials, the NHS’ next step is to “define” a “pathway” for those who decide to detransition, since there is currently no official guidance on how to care for such individuals. Their work will involve examining the proportion of patients who detransition, and their reasons for detransitioning, The Telegraph reported.
The plan involves the creation of six new clinics by 2026 specialized to care for minors struggling with their biological sex.
Despite this impending reform, the NHS is set to begin clinical trials of puberty blockers for minors, since Dr. Cass’ report cited lack of long-term studies as a reason that puberty blockers should not be prescribed to minors.
Critics have warned that these trials are “ethically unjustifiable,” with the warning that they “pose the very real risk of the NHS sacrificing the otherwise good health of vulnerable children and causing them grave physical harm in the name of research.”
Lucy Marsh of the Family Education Trust has called upon the NHS to address the roots of gender dysphoria and has decried its planned trials of administering puberty blockers to teenagers as “unethical” and “dangerous.”
‘We do not need more gender clinics, instead the NHS should be looking at the root causes of gender dysphoria including mental health issues, autism, sexual abuse and issues within the family,” said Marsh, according to The Daily Mail.
“It is not ‘kind’ to lead children down a pathway that leads to irreversible harm and destroys families,” she said, adding that it is a “a huge waste of taxpayer’s money to roll out gender clinics to every area of England.”
Transgender hormonal and surgical interventions are known to cause lifelong mental and physical damage and to exacerbate psychological issues in those subjected to them.
Studies find that more than 80 percent of children experiencing gender dysphoria outgrow it on their own by late adolescence, and that even full “reassignment” surgery often fails to resolve gender-confused individuals’ heightened tendency to engage in self-harm and suicide – and may even exacerbate it, including by reinforcing their confusion and neglecting the actual root causes of their mental strife.
Many oft-ignored detransitioners have attested to the physical and mental harm of reinforcing gender confusion as well as to the bias and negligence of the medical establishment on the subject, many of whom take an activist approach to their profession and begin cases with a predetermined conclusion that “transitioning” is the best solution.
Health
All 12 Vaccinated vs. Unvaccinated Studies Found the Same Thing: Unvaccinated Children Are Far Healthier
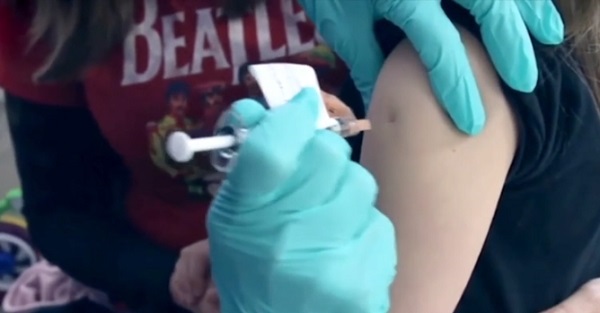
I joined Del Bigtree in studio on The HighWire to discuss what the data now make unavoidable: the CDC’s 81-dose hyper-vaccination schedule is driving the modern epidemics of chronic disease and autism.
This was not a philosophical debate or a clash of opinions. We walked through irrefutable, peer-reviewed evidence showing that whenever vaccinated and unvaccinated children are compared directly, the unvaccinated group is far healthier—every single time.
Reanalyzing the Largest Vaccinated vs. Unvaccinated Birth-Cohort Study Ever Conducted
At the center of our discussion was our peer-reviewed reanalysis of the Henry Ford Health System vaccinated vs. unvaccinated birth-cohort study (Lamerato et al.)—the largest and most rigorous comparison of its kind ever conducted.
|
The original authors relied heavily on Cox proportional hazards models, a time-adjusted approach that can soften absolute disease burden. Even so, nearly all chronic disease outcomes were higher in vaccinated children.
Our reanalysis used direct proportional comparisons, stripping away the smoothing and revealing the full magnitude of the signal.
- All 22 chronic disease categories favored the unvaccinated cohort when proportional disease burden was examined
- Cancer incidence was 54% higher in vaccinated children (0.0102 vs. 0.0066)
- When autism-associated conditions were grouped appropriately—including autism, ADHD, developmental delay, learning disability, speech disorder, neurologic impairment, seizures, and related diagnoses—the vaccinated cohort showed a 549% higher odds of autism-spectrum–associated clinical outcomes
The findings are internally consistent, biologically coherent, and concordant with every prior vaccinated vs. unvaccinated study, all of which show drastically poorer health outcomes among vaccinated children
The 12 Vaccinated vs. Unvaccinated Studies Regulators Ignore
In the McCullough Foundation Autism Report, we compiled all 12 vaccinated vs. unvaccinated pediatric studies currently available. These studies span different populations, countries, study designs, and data sources.
Every single one reports the same overall pattern. Across all 12 studies, unvaccinated children consistently exhibit substantially lower rates of chronic disease, including:
- Autism and other neurodevelopmental disorders
- ADHD, tics, learning and speech disorders
- Asthma, allergies, eczema, and autoimmune conditions
- Chronic ear infections, skin disorders, and gastrointestinal illness
This level of consistency across independent datasets is precisely what epidemiology looks for when assessing causality. It also explains why no federal agency has ever conducted—or endorsed—a fully vaccinated vs. fully unvaccinated safety study.
Flu Shot Failure
We also addressed the persistent failure of seasonal influenza vaccination.
A large Cleveland Clinic cohort study of 53,402 employees followed participants during the 2024–2025 respiratory viral season and found:
- 82.1% of employees were vaccinated against influenza
- Vaccinated individuals had a 27% higher adjusted risk of influenza compared with the unvaccinated state (HR 1.27; 95% CI 1.07–1.51; p = 0.007)
- This corresponded to a negative vaccine effectiveness of −26.9% (95% CI −55.0 to −6.6%), meaning vaccination was associated with increased—not reduced—risk of influenza
When vaccination exposure increases, chronic disease, neurodevelopmental disorders, and inflammatory illness increase with it. When children are unvaccinated, they are measurably healthier across virtually every outcome that matters.
The science needed to confront the chronic disease and autism epidemics already exists. What remains is the willingness to acknowledge it.
Epidemiologist and Foundation Administrator, McCullough Foundation
Support our mission: mcculloughfnd.org
Please consider following both the McCullough Foundation and my personal account on X (formerly Twitter) for further content.
FOCAL POINTS (Courageous Discourse) is a reader-supported publication.
To receive new posts and support my work, consider becoming a free or paid subscriber.
Daily Caller
Ex-FDA Commissioners Against Higher Vaccine Standards Took $6 Million From COVID Vaccine Makers


From the Daily Caller News Foundation
By Emily Kopp
The FDA old guard criticized the new leadership in a Dec. 3 New England Journal of Medicine (NEJM) letter over a higher regulatory bar for vaccines, namely the expectation that most new vaccine approvals will require randomized clinical trials, arguing it could hamper the market.
“Insisting on long, expensive outcomes studies for every updated formulation would delay the arrival of better-matched vaccines when new outbreaks emerge or when additional groups of patients could benefit,” the former commissioners wrote. “Abandoning the existing methods won’t ‘elevate vaccine science’ … It will subject vaccines to a substantially higher and more subjective approval bar.”
But while the former commissioners disclosed their conflicts of interest to the medical journal — per standard practice in scientific publishing — reporters didn’t relay them to the broader public in reports in the Washington Post, STAT News and CNN.
The headlines about a bipartisan rebuke from former occupants of FDA’s highest office give the impression that the Trump administration is contravening established science, but closer inspection reveals a revolving door between pharmaceutical corporations and the agencies overseeing them.
Three of the signatories have received payments totaling $6 million from manufacturers or former manufacturers of COVID vaccines.
Scott Gottlieb has received $2.1 million in cash and stock from his position on the Pfizer board of directors, where he has advised on ethics and regulatory compliance since 2019, according to company filings to the Securities and Exchange Commission. Stephen Ostroff has received $752,310 from Pfizer in consulting fees since 2020, according to OpenPayments.
Mark McClellan has received $3.3 million from Johnson & Johnson as a member of the board of directors since 2013, SEC filings also show. McClellan also consults for the new pharmaceutical arm of the alternative investment management company Blackstone, which invested $750 million in Moderna in April 2025.
Gottlieb and McClellan did not respond to requests for comment. Ostroff could not be reached for comment.
FDA Center for Biologics Evaluation and Research Director Vinay Prasad outlined the higher standards and shared the results of an internal analysis validating 10 reports of children’s deaths following the COVID-19 vaccine in a Nov. 28 memo to staff. He called for introspection and reform at the agency.
The NEJM letter criticizes Prasad for cracking down on a practice called “immunobridging” that infers vaccine efficacy from laboratory tests rather than assessing it through real-world reductions in disease or death. The FDA under the Biden administration expanded COVID vaccines to children using this “immunobridging” technique, extrapolating vaccine efficacy from adults to children based on antibody levels.
Norman Sharpless — who in addition to previously serving as acting FDA commissioner also served as the head of the National Institutes of Health’s National Cancer Institute — consults for Tempus, a company that collaborates with COVID vaccine maker BioNTech. He has helped steer $70 million in investments in biotech through a venture capital firm he founded in November 2024. Sharpless also disclosed $26,180 in payments in 2024 from Chugai Pharmaceutical, a Japanese pharmaceutical company that markets mRNA technology among other drugs, on OpenPayments.
“I was grateful for the opportunity to serve as NCI Director and Acting FDA Commissioner in the first Trump Administration, and strongly support many of the things President Trump is trying to do in the current Administration,” Sharpless said in an email.
Margaret Hamburg, another former FDA commissioner and signatory of the NEJM letter, has since 2020 earned $2.8 million as a member of the board of Alnylam Pharmaceuticals, which markets RNA interference (RNAi) technology.
Hamburg did not respond to a message on LinkedIn.
Most signatories disclosed income from biotech companies testing experimental cancer treatments. These products could face tighter scrutiny under Prasad, a hematologist-oncologist long wary of rubberstamping pricey oncology drugs — which Prasad points out often cause some toxicity — without plausible evidence of an improvement in quality of life or survival.
The former FDA commissioners disclosed ties to Sermonix Pharmaceuticals Inc.; OncoNano Medicine; incyclix; Nucleus Radiopharma; and N-Power, a contractor that runs oncology clinical trials.
Andrew von Eschenbach, who like Sharpless formerly served both as FDA commissioner and the head of the National Cancer Institute, disclosed stock in HistoSonics, a company with investments from Bezos Expeditions and Thiel Bio seeking FDA approval for ultrasound technology targeted at tumors.
Some FDA commissioners who signed onto the letter opposing changes to vaccine approvals have ties to biotechnology investment firms, namely McClellan, who consults Arsenal Capital; Janet Woodcock, who consults RA Capital Management; and Robert Califf, who owns stock in Population Health Partners.
Califf did not respond to an email requesting comment. Woodcock did not respond to requests for comment sent to two medical research advocacy groups with Woodcock on the board. Eschenbach did not respond to a LinkedIn message.
The two signatories without pharmaceutical ties may find their judgement challenged by the FDA investigation into COVID-19 vaccine deaths, having either implemented or formally defended the Biden administration’s headlong expansion of vaccines and boosters to healthy adults and children.
David Kessler executed Biden’s vaccination policy as chief science officer at the Department of Health and Human Services, helping to secure deals for shots with Pfizer and Moderna.
Meanwhile Jane Henney chaired a National Academies of Sciences, Engineering, and Medicine report published in October 2025 that praised the performance of FDA and Centers for Disease Control and Prevention (CDC) vaccine surveillance during the pandemic — underwritten with CDC funding.
That assessment clashes with that of a Senate report, citing internal documents from FDA, finding that CDC never updated its vaccine surveillance tool “V-Safe” to include cardiac symptoms, despite naming myocarditis as a potential adverse event by October 2020, and that top officials in the Biden administration delayed warning pediatricians and other providers about the risk of myocarditis after their approval in some children in May 2021, months after Israeli health officials first detected it in February 2021. The Senate investigation named Woodcock, a signatory of the NEJM letter, as one of the FDA officials who slow-walked the warning.
-

 Crime2 days ago
Crime2 days agoBrown University shooter dead of apparent self-inflicted gunshot wound
-

 Alberta1 day ago
Alberta1 day agoAlberta’s new diagnostic policy appears to meet standard for Canada Health Act compliance
-

 Censorship Industrial Complex1 day ago
Censorship Industrial Complex1 day agoCanadian university censors free speech advocate who spoke out against Indigenous ‘mass grave’ hoax
-

 Business18 hours ago
Business18 hours agoGeopolitics no longer drives oil prices the way it used to
-

 Business18 hours ago
Business18 hours agoArgentina’s Milei delivers results free-market critics said wouldn’t work
-

 Health1 day ago
Health1 day agoRFK Jr reversing Biden-era policies on gender transition care for minors
-

 Business2 days ago
Business2 days agoTrump signs order reclassifying marijuana as Schedule III drug
-

 Business18 hours ago
Business18 hours agoDeadlocked Jury Zeroes In on Alleged US$40 Million PPE Fraud in Linda Sun PRC Influence Case














