COVID-19
Treat COVID-19 like the flu: new CDC guidelines

Press release from the CDC
CDC updates and simplifies respiratory virus recommendations
Recommendations are easier to follow and help protect those most at risk
CDC released today (Friday, March 1, 2024) updated recommendations for how people can protect themselves and their communities from respiratory viruses, including COVID-19. The new guidance brings a unified approach to addressing risks from a range of common respiratory viral illnesses, such as COVID-19, flu, and RSV, which can cause significant health impacts and strain on hospitals and health care workers. CDC is making updates to the recommendations now because the U.S. is seeing far fewer hospitalizations and deaths associated with COVID-19 and because we have more tools than ever to combat flu, COVID, and RSV.
“Today’s announcement reflects the progress we have made in protecting against severe illness from COVID-19,” said CDC Director Dr. Mandy Cohen. “However, we still must use the commonsense solutions we know work to protect ourselves and others from serious illness from respiratory viruses—this includes vaccination, treatment, and staying home when we get sick.”
As part of the guidance, CDC provides active recommendations on core prevention steps and strategies:
- Staying up to date with vaccination to protect people against serious illness, hospitalization, and death. This includes flu, COVID-19, and RSV if eligible.
- Practicing good hygiene by covering coughs and sneezes, washing or sanitizing hands often, and cleaning frequently touched surfaces.
- Taking steps for cleaner air, such as bringing in more fresh outside air, purifying indoor air, or gathering outdoors.
When people get sick with a respiratory virus, the updated guidance recommends that they stay home and away from others. For people with COVID-19 and influenza, treatment is available and can lessen symptoms and lower the risk of severe illness. The recommendations suggest returning to normal activities when, for at least 24 hours, symptoms are improving overall, and if a fever was present, it has been gone without use of a fever-reducing medication.
Once people resume normal activities, they are encouraged to take additional prevention strategies for the next 5 days to curb disease spread, such as taking more steps for cleaner air, enhancing hygiene practices, wearing a well-fitting mask, keeping a distance from others, and/or getting tested for respiratory viruses. Enhanced precautions are especially important to protect those most at risk for severe illness, including those over 65 and people with weakened immune systems. CDC’s updated guidance reflects how the circumstances around COVID-19 in particular have changed. While it remains a threat, today it is far less likely to cause severe illness because of widespread immunity and improved tools to prevent and treat the disease. Importantly, states and countries that have already adjusted recommended isolation times have not seen increased hospitalizations or deaths related to COVID-19.
While every respiratory virus does not act the same, adopting a unified approach to limiting disease spread makes recommendations easier to follow and thus more likely to be adopted and does not rely on individuals to test for illness, a practice that data indicates is uneven.
“The bottom line is that when people follow these actionable recommendations to avoid getting sick, and to protect themselves and others if they do get sick, it will help limit the spread of respiratory viruses, and that will mean fewer people who experience severe illness,” National Center for Immunization and Respiratory Diseases Director Dr. Demetre Daskalakis said. “That includes taking enhanced precautions that can help protect people who are at higher risk for getting seriously ill.”
The updated guidance also includes specific sections with additional considerations for people who are at higher risk of severe illness from respiratory viruses, including people who are immunocompromised, people with disabilities, people who are or were recently pregnant, young children, and older adults. Respiratory viruses remain a public health threat. CDC will continue to focus efforts on ensuring the public has the information and tools to lower their risk or respiratory illness by protecting themselves, families, and communities.
This updated guidance is intended for community settings. There are no changes to respiratory virus guidance for healthcare settings.
Freedom Convoy
Court Orders Bank Freezing Records in Freedom Convoy Case
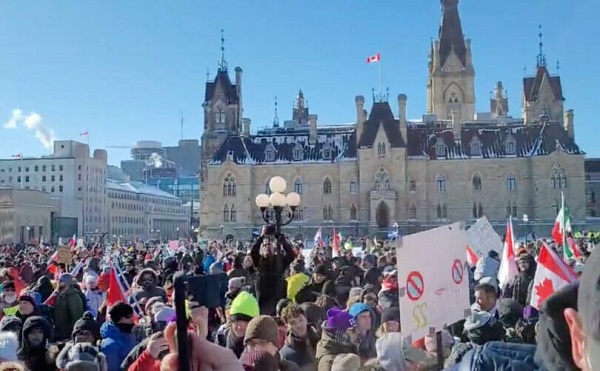
|
A Canadian court has ordered the release of documents that could shed light on how federal authorities and law enforcement worked together to freeze the bank accounts of a protester involved in the Freedom Convoy.
Both the RCMP and TD Bank are now required to provide records related to Evan Blackman, who took part in the 2022 demonstrations and had his accounts frozen despite not being convicted of any crime at the time.
The Justice Centre for Constitutional Freedoms (JCCF) announced the Ontario Court of Justice ruling. The organization is representing Blackman, whose legal team argues that the actions taken against him amounted to a serious abuse of power.
“The freezing of Mr. Blackman’s bank accounts was an extreme overreach on the part of the police and the federal government,” said his lawyer, Chris Fleury. “These records will hopefully reveal exactly how and why Mr. Blackman’s accounts [were] frozen.”
Blackman was arrested during the mass protests in Ottawa, which drew thousands of Canadians opposed to vaccine mandates and other pandemic-era restrictions.
Although he faced charges of mischief and obstructing police, those charges were dismissed in October due to a lack of evidence. Despite this, prosecutors have appealed, and a trial is set to begin on August 14.
At the height of the protests, TD Bank froze three of Blackman’s accounts following government orders issued under the Emergencies Act. Then-Prime Minister Justin Trudeau had invoked the act to grant his government broad powers to disrupt the protest movement, including the unprecedented use of financial institutions to penalize individuals for their support or participation.
In 2024, a Federal Court Justice ruled that Trudeau’s decision to invoke the act had not been justified.
Blackman’s legal team plans to use the newly released records to demonstrate the extent of government intrusion into personal freedoms.
According to the JCCF, this case may be the first in Canada where a criminal trial includes a Charter challenge over the freezing of personal bank accounts under emergency legislation.
|
COVID-19
FDA requires new warning on mRNA COVID shots due to heart damage in young men
From LifeSiteNews
Pfizer and Moderna’s mRNA COVID shots must now include warnings that they cause ‘extremely high risk’ of heart inflammation and irreversible damage in males up to age 24.
The Trump administration’s Food and Drug Administration (FDA) announced it will now require updated safety warnings on mRNA COVID-19 shots to include the “extremely high risk” of myocarditis/pericarditis and the likelihood of long-term, irreversible heart damage for teen boys and young men up to age 24.
The required safety updates apply to Comirnaty, the mRNA COVID shot manufactured by Pfizer Inc., and Spikevax, the mRNA COVID shot manufactured ModernaTX, Inc.
According to a press release, the FDA now requires each of those manufacturers to update the warning about the risks of myocarditis and pericarditis to include information about:
- the estimated unadjusted incidence of myocarditis and/or pericarditis following administration of the 2023-2024 Formula of mRNA COVID-19 shots and
- the results of a study that collected information on cardiac magnetic resonance imaging (cardiac MRI) in people who developed myocarditis after receiving an mRNA COVID-19 injection.
The FDA has also required the manufacturers to describe the new safety information in the adverse reactions section of the prescribing information and in the information for recipients and caregivers.
Additionally, the fact sheets for healthcare providers and for recipients and caregivers for Moderna COVID-19 shot and Pfizer-BioNTech COVID-19 shot, which are authorized for emergency use in individuals 6 months through 11 years of age, have also been updated to include the new safety information in alignment with the Comirnaty and Spikevax prescribing information and information for recipients and caregivers.
In a video published on social media, Dr. Vinay Prasad, director of the Center for Biologics Evaluation & Research Chief Medical and Scientific Officer, explained the alarming reasons for the warning updates.
While heart problems arose in approximately 8 out of 1 million persons ages 6 months to 64 years following reception of the cited shots, that number more than triples to 27 per million for males ages 12 to 24.
Prasad noted that multiple studies have arrived at similar findings.
-

 International2 days ago
International2 days agoChicago suburb purchases childhood home of Pope Leo XIV
-

 Daily Caller2 days ago
Daily Caller2 days agoBlackouts Coming If America Continues With Biden-Era Green Frenzy, Trump Admin Warns
-

 Daily Caller2 days ago
Daily Caller2 days ago‘I Know How These People Operate’: Fmr CIA Officer Calls BS On FBI’s New Epstein Intel
-

 Crime23 hours ago
Crime23 hours agoTrump supporters cry foul after DOJ memo buries the Epstein sex trafficking scandal
-

 Daily Caller22 hours ago
Daily Caller22 hours agoTrump Issues Order To End Green Energy Gravy Train, Cites National Security
-

 Daily Caller15 hours ago
Daily Caller15 hours agoUSAID Quietly Sent Thousands Of Viruses To Chinese Military-Linked Biolab
-

 Addictions15 hours ago
Addictions15 hours ago‘Over and over until they die’: Drug crisis pushes first responders to the brink
-

 Business1 day ago
Business1 day agoPrime minister can make good on campaign promise by reforming Canada Health Act