COVID-19
Apply for Canada Emergency Response Benefit (CERB) with CRA

The Government of Canada is issuing payments to workers residing in Canada who have lost income or self-employment income for reasons related to COVID-19.
You can apply for this benefit through either the Canada Revenue Agency (CRA) or Service Canada, but not both.
By applying, you are giving consent to the CRA to use your tax information for the purposes of administering and enforcing the CERB, and are agreeing that your information, including tax information, may be shared with Employment and Social Development Canada.A.
Click here to start your CERB application. If you haven’t already, you may be asked to setup your direct deposit with CRA https://www.canada.ca/en/revenue-agency/services/benefits/apply-for-cerb-with-cra.html


Copied off of the CRA website.
Who can apply
To be eligible, you must meet the following requirements:
- You reside in Canada
- You are 15 years old or more when you apply
- For your first CERB application:
- You have stopped or will stop working due to reasons related to COVID-19
- For at least 14 days in a row for the period you are applying for, you will not receive:
- employment income
- self-employment income
- provincial or federal benefits related to maternity or paternity leave
- For your subsequent CERB applications:
- You continue to not work due to reasons related to COVID-19
- For the 4 week period you are applying for, you will not receive:
- employment income;
- self-employment income; or
- provincial or federal benefits related to maternity or paternity leave.
- You have not quit your job voluntarily
- You did not apply for, nor receive, CERB or EI benefits from Service Canada for the same eligibility period
- You earned a minimum of $5,000 income in the last 12 months or in 2019 from one or more of the following sources:
- employment income
- self-employment income
- provincial or federal benefits related to maternity or paternity leave
Raoul Bhatt
https://instagram.com/raoul
COVID-19
Nearly Half of “COVID-19 Deaths” Were Not Due to COVID-19 – Scientific Reports Journal


 Nicolas Hulscher, MPH
Nicolas Hulscher, MPH
45.3% of “COVID-19 deaths” in Greece had no symptoms — exposing the coordinated PSYOP deployed to maximize fear and enforce mass compliance with draconian control measures.
The study titled “Deaths “due to” COVID-19 and deaths “with” COVID-19 during the Omicron variant surge, among hospitalized patients in seven tertiary-care hospitals, Athens, Greece” was just published in the journal Scientific Reports:
Abstract
In Greek hospitals, all deaths with a positive SARS-CoV-2 test are counted as COVID-19 deaths. Our aim was to investigate whether COVID-19 was the primary cause of death, a contributing cause of death or not-related to death amongst patients who died in hospitals during the Omicron surge and were registered as COVID-19 deaths. Additionally, we aimed to analyze the factors associated with the classification of these deaths. We retrospectively re-viewed all in-hospital deaths, that were reported as COVID-19 deaths, in 7 hospitals, serving Athens, Greece, from January 1, 2022, until August 31, 2022. We retrieved clinical and laboratory data from patient records. Each death reported as COVID-19 death was characterized as: (A) death “due to” COVID-19, or (B) death “with” COVID-19. We reviewed 530 in-hospital deaths, classified as COVID-19 deaths (52.4% males; mean age 81.7 ± 11.1 years). We categorized 290 (54.7%) deaths as attributable or related to COVID-19 and in 240 (45.3%) deaths unrelated to COVID-19. In multivariable analysis The two groups differed significantly in age (83.6 ± 9.8 vs. 79.9 ± 11.8, p = 0.016), immunosuppression history (11% vs. 18.8%, p = 0.027), history of liver disease (1.4% vs. 8.4%, p = 0.047) and the presence of COVID-19 symptoms (p < 0.001). Hospital stay was greater in persons dying from non-COVID-19 related causes. Among 530 in-hospital deaths, registered as COVID-19 deaths, in seven hospitals in Athens during the Omicron wave, 240 (45.28%) were reassessed as not directly attributable to COVID-19. Accuracy in defining the cause of death during the COVID-19 pandemic is of paramount importance for surveillance and intervention purposes.
Key Findings:
Massive Overcounting of COVID-19 Deaths
- Out of 530 hospital deaths registered as COVID-19 deaths, only 290 (54.7%) were actually caused by COVID-19.
- 240 deaths (45.3%) were found to be completely unrelated to COVID-19 — patients died with a positive PCR test, but showed no symptoms, required no COVID-specific treatment, and died of clearly unrelated causes.
Death Certificate Inaccuracy
- Of the 204 certificates listing COVID-19 as the direct cause of death, only 132 (64.7%) were confirmed as such after clinical review.
- Of the 324 certificates listing COVID-19 as a contributing factor, only 86 (26.5%) were found to be truly related.
Hospital-Acquired Infections Misclassified
- Patients infected during hospitalization were significantly more likely to be misclassified as COVID-19 deaths (OR: 2.3, p = 0.001).
Younger Age and Severe Comorbidities Associated with Misclassification
- Patients who died “with” COVID-19 were younger, more likely to be immunosuppressed, have end-stage liver disease, or be admitted for other causes.
Symptoms and Treatments Differed Sharply
Patients who died “due to” COVID-19 were more likely to:
- Exhibit classic symptoms: hypoxia (44.1%), shortness of breath, fever, and cough
- Require oxygen support (93.4% vs. 66.9%) and receive COVID-specific therapies:
- Remdesivir (5-day course: 61.9% vs. 35.2%)
- Dexamethasone (81.7% vs. 40.7%)
Study Strengths
This study went far beyond death certificate coding, implementing a rigorous, multi-source clinical audit:
- Full medical chart reviews: Included physician notes, lab data, imaging, and treatment records.
- Attending physician interviews: Structured questionnaires captured real-time clinical insights from those who treated the patients.
- Dual independent expert assessments: Two experienced infectious disease specialists (each with >2,500 COVID cases) reviewed each case independently for classification accuracy.
This study found that nearly half of all registered COVID-19 deaths during the Omicron wave in Greece were misclassified, with no clinical evidence linking them to COVID-19 as the true cause. Given that similar death coding practices were employed across Western nations, it is reasonable to conclude that COVID-19 death counts were artificially inflated to a comparable degree elsewhere.
This drastic inflation of death counts aligns with what many now understand to be a coordinated psychological operation (PSYOP)—designed to instill fear and maximize compliance with draconian pandemic measures such as lockdowns, mask mandates, and mass mRNA injection campaigns.
It is this weaponization of fear that has prompted criminal referrals in seven U.S. states, triggering active criminal investigations into top COVID-19 officials for terrorism, murder and racketeering:
BREAKING – The Pandemic Justice Phase Begins as Criminal Investigations Commence |
||||||
|
||||||
|
By Nicolas Hulscher, MPH
|
||||||
|
Epidemiologist and Foundation Administrator, McCullough Foundation
Please consider following both the McCullough Foundation and my personal account on X (formerly Twitter) for further content.
2025 Federal Election
Before the Vote: Ask Who’s Defending Our Health

From the World Council for Health Canada
The health of Canadians has been compromised by government-mandated COVID-19 injections. The upcoming federal election is an opportunity to demand change and accountability. As you decide which candidate or party is most committed to defending the health of yourself and your family, please consider the following:
The Injections Were Never What They Claimed
The Canadian government successfully mandated the COVID-19 injections by labeling them “safe and effective vaccines.” These products are still being promoted and administered across the country. However, the truth is:
- They are not vaccines: Click Here
- They are not safe: Click Here
- They do not prevent infection or transmission.
- Evidence shows they increase the risk of COVID-19 disease and death: Click Here
These Products Contain Multiple Mechanisms of Harm
- They cause injury through multiple biological mechanisms: Click Here
- They have surpassed all vaccines in recorded history—for all infections, for all of the past thirty years combined—in causing deaths and injuries: Click Here
- They are chemically contaminated and adulterated with DNA: Click Here
- In Pfizer’s case, fraud is evident: the DNA contamination includes genetic engineering tools derived from the SV40 virus, associated with cancer risks: Click Here
This Election, We Must Demand Accountability
Insist that to have your vote, candidates must:
- Denounce the COVID-19 “vaccines.”
- Support a full halt to their manufacturing and administration.
- Uphold informed consent, scientific integrity, and bodily autonomy.
Your voice is important. Use it to reject censorship, harm, and medical coercion.
-

 International1 day ago
International1 day agoPope Francis has died aged 88
-

 International1 day ago
International1 day agoPope Francis Dies on Day after Easter
-

 International1 day ago
International1 day agoJD Vance was one of the last people to meet Pope Francis
-
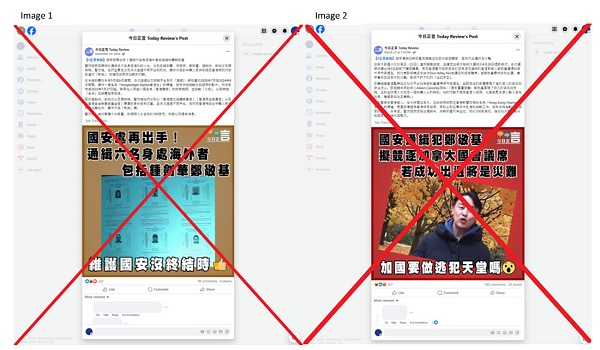
 2025 Federal Election15 hours ago
2025 Federal Election15 hours agoOttawa Confirms China interfering with 2025 federal election: Beijing Seeks to Block Joe Tay’s Election
-

 2025 Federal Election1 day ago
2025 Federal Election1 day agoCarney’s budget means more debt than Trudeau’s
-

 2025 Federal Election15 hours ago
2025 Federal Election15 hours agoReal Homes vs. Modular Shoeboxes: The Housing Battle Between Poilievre and Carney
-

 COVID-1915 hours ago
COVID-1915 hours agoNearly Half of “COVID-19 Deaths” Were Not Due to COVID-19 – Scientific Reports Journal
-

 2025 Federal Election14 hours ago
2025 Federal Election14 hours agoHow Canada’s Mainstream Media Lost the Public Trust








