COVID-19
There are no licensed COVID shots for kids under 12 – but CDC wants babies to get 3 Pfizer shots by 9 months
From LifeSiteNews
By and
“This is an insult to people’s intelligence,” she said, “I pray that parents will have the good sense to say no to these dangerous and unnecessary shots for babies.”
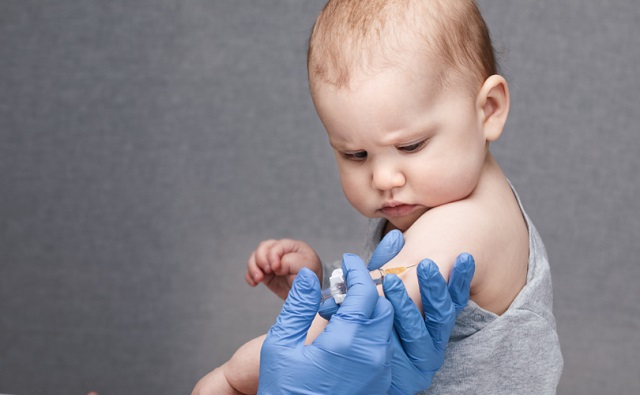
Nine-month-old babies must receive multiple doses of an unlicensed mRNA COVID-19 vaccine to be considered “up to date” with their COVID-19 vaccination, according to the Centers for Disease Control and Prevention (CDC).
The CDC’s updated guidance, issued August 30, states that children – as young as 6 months old – should get either two doses of the 2024-2025 Moderna vaccine or three doses of the 2024-2025 Pfizer-BioNTech vaccine.
If getting the new Pfizer shot, the baby is supposed to receive the first dose at 6 months, the second dose three weeks later and the third dose at least eight weeks after the second dose – meaning, that by 9 months old, babies are supposed to have received three Pfizer shots.
If getting the latest Moderna shot, the CDC recommends babies get the first dose at age 6 months and the second dose a month later.
The latest Pfizer and Moderna COVID-19 shots for children under 12 are unlicensed in the U.S. The U.S. Food and Drug Administration (FDA) has granted only emergency use authorization (EUA) for the vaccines.
Children’s Health Defense (CHD) CEO Mary Holland told The Defender, “The earlier COVID shots have been proven unsafe and ineffective. Now we’re asked to believe that newer versions are miraculously safe and effective?”
“This is an insult to people’s intelligence,” she said, “I pray that parents will have the good sense to say no to these dangerous and unnecessary shots for babies.”
As of July 28, 37,814 deaths following COVID-19 vaccination had been reported to VAERS, the Vaccine Adverse Event Reporting System, run by the FDA and CDC.
Of those, 187 reports were for children and teens under 18. Nearly 13,000 reports listed the age as “unknown.”
VAERS analyst and expert Albert Benavides recently told The Defender he believes VAERS is “throttling” and underreporting deaths of all ages following COVID-19 vaccination.
Meanwhile, the CDC continues to tell the public that COVID-19 vaccines are “safe and effective.”
CDC ‘absolutely misleading’ public on safety of EUA vaccines
Holland said the CDC is “absolutely misleading” the public by asserting that COVID-19 EUA vaccines are safe and effective because EUA vaccines are not held to the same safety or efficacy standards as licensed vaccines.
“By law,” she explained, “EUA products ‘may be effective,’ and they have not undergone the safety testing required to permit licensing.”
“This is one more horrific example of the CDC putting profits before people and acting as an unethical arm of Big Pharma’s marketing operation,” Holland added.
CHD Chief Scientific Officer Brian Hooker agreed. “It is criminal that these untested vaccines are being recommended to infants and children, especially given the fraudulent tactics to market them to an unsuspecting public,” Hooker told The Defender.
There’s no licensed COVID vaccine for kids under 12
There are still no licensed COVID-19 vaccines available for children under 12, Hooker said – so all COVID-19 vaccines given to young kids are EUA products.
The FDA’s website on EUA for medical products states that EUA vaccines only have to meet the standard of “may be effective” as long as if, “based on the totality of the scientific evidence, it is reasonable to believe that the product may be effective for the specified use.”
“The ‘may be effective’ standard for EUAs provides for a lower level of evidence than the ‘effectiveness’ standard that FDA uses for product approvals,” the website states.
Before a vaccine can be fully licensed, the vaccine maker typically is required to conduct numerous clinical trials to demonstrate that the product is safe. However, the safety requirements for EUA are more flexible.
According to the FDA:
The amount and type(s) of safety information that FDA recommends be submitted as part of a request for an EUA will differ depending upon a number of factors, including whether the product is approved for another indication and, in the case of an unapproved product, the product’s stage of development.
Despite this, the first statement on the CDC’s “6 Things to Know about COVID-19 Vaccination for Children” says, “COVID-19 vaccination for children is safe.”
Risks outweigh benefits for kids
Hooker said the CDC’s actions are especially problematic as, historically, the meaning of “safe” has been interpreted by regulatory authorities as meaning that the benefits of a drug outweigh its risks.
“With the risk to children of dying from a COVID-19 infection being statistically zero, it is unclear if there is any benefit,” he said.
Meanwhile, the CDC still claims that “while adverse reactions are rare, the benefits of COVID-19 vaccination outweigh the known risks of COVID-19 and possible severe complications.”
Pfizer fact sheet more forthcoming about risks
For licensed vaccines, the CDC typically provides an official vaccine information statement (VIS) that describes the vaccine’s risks and potential benefits.
According to the CDC website, “Federal law requires that healthcare staff provide a VIS to a patient, parent, or legal representative before each dose of certain vaccines.”
However, for EUA COVID-19 vaccines, the CDC directs people to “fact sheets” – produced by the vaccine manufacturer, not the CDC, and authorized by the FDA – which detail the product’s risks and benefits.
There is no federal law requiring healthcare providers to share these fact sheets with patients, or parents of minors, before a COVID-19 vaccination.
“Pfizer’s own ‘fact sheet’ for its latest COVID-19 vaccine appears to give a more accurate picture [of the vaccine’s risks] than the CDC’s own websites,” Hooker said. “Shouldn’t the CDC be more a watchdog than Pfizer?”
For example, Pfizer’s fact sheet states, “A product authorized for emergency use has not undergone the same type of review by FDA as an FDA-approved product.”
The Pfizer fact sheet also acknowledges that its vaccine “may not protect everyone” and that reported side effects associated with the Pfizer vaccines include myocarditis and pericarditis.
Hooker pointed out that research has shown that vaccine-induced myocarditis, inflammation of the heart, and pericarditis, inflammation of the tissue surrounding the heart, can be fatal.
He urged parents to “read between the lines” when assessing the CDC’s COVID-19 vaccination recommendation for babies and children.
“Most of all,” he added, “use common sense to decide if the CDC’s and the FDA’s logic is sound.”
This article was originally published by The Defender – Children’s Health Defense’s News & Views Website under Creative Commons license CC BY-NC-ND 4.0. Please consider subscribing to The Defender or donating to Children’s Health Defense.
Bruce Dowbiggin
The Covid 19 Disaster: When Do We Get The Apologies?

Breaking: Drs. Bonnie Henry and Theresa Tam have been appointed to the Order of Canada in recognition of their role in the country’s response to the COVID-19 pandemic.
And so the game of covid liar’s poker has more winners. It’s like awarding the captain of the Titanic the Nobel Prize for his work on floatation. As we now know these two— and the other WHO finger puppets in Canada— made the Covid 19 episode worse, not better, with their prescription for panic, positives and punishment. Even as they knew the truth about the limits of the virus and the efficacy of vaccines they continued to spew fallacious PCR data on the extent of the sickness and who was at risk.
Put simply, to protect vulnerable seniors they said kids were also at great risk. Which was unconscionable.
In this they encouraged Justin Trudeau in his worst instincts, combining his father’s insouciant disregard for civil rights (sending in the police) with his mother’s mental stability. Propped up by Team Tam and its U.S. allies such as Anthony Fauci, this hysteria peaked with a sequestered PM crushing the Truckers Convoy’s vaccine protest with emergency measures and destruction of civil liberties.
Lest you wonder, this overreach was recognized at the time. Justice Maclean wrote at the trial of Convoy organizers, “Defendants & other persons remain at liberty to engage in a peaceful, lawful & safe protest”. On Feb. 16, he continued a no-honking order, again writing: “Defendants & other persons remain at liberty to engage in a peaceful, lawful & safe protest.”
The leaders of the Convoy, lynched by Canadian media’s phoney claims of right-wing American interference, are still fighting jail time on charges of nuisance. While violent criminals are routinely released on bail or absolved.
Justice Richard Mosley later concluded that while the convoy was a disruption of public order, it didn’t constitute a national emergency and invoking the act “does not bear the hallmarks of reasonableness — justification, transparency and intelligibility.” But in real time Team Tam made no attempts to correct the wilder misgivings about Covid (lockdowns, mandatory vaccines). Trudeau was given a hall pass. Needless to say the purchased media made things infinitely worse regurgitating these mistakes.
In short, they knew better but hid the truth. But why pick on Henry and Tam? Under Trudeau and his wingman Jagmeet Singh this was the golden age of lies and prevarications in Canada and the U.S. No apologies were ever offered when the truth emerged.
As we’ve noted before, Trudeau cried with a teddy bear carefully positioned over 751 alleged unmarked graves in a known Catholic cemetery that the local Cowessess band abandoned. The Liberal government knew the claim of 215 “children’s graves” was false, and still ran with it to get Trudeau his photo-op. Naturally the CBC Media Party played (and still plays) accomplice in this farce as the Canadian flag was lowered to half-mast for six months and Trudeau ratted out Canada at the UN as a genocidal state.
There were more, plenty more Trudeau scandals that media endorsed and then stood by even as the truth was revealed. SNC Lavalin. We Charity. Arrive Can app. Firing indigenous justice minister. Chinese drug infiltration/ money laundering. Nazi Celebrated in Parliament. Welcome To Canada immigration. Nova Scotia massacre. McKinsey Consultation. Blackface. And so on.
And were there apologies when it came time to make the Trudeau Liberals accountable? No, they staged a media circus over Donald Trump’s assertion of 51st state. All the fake news and deliberate lies went poof, allowing Mark Carney to seamlessly assume the PM job.

Lest We Forget Pt. 2 it was not exclusive to Canada. As we are now learning: Barack Obama and Joe Biden sat in an August 3, 2016 Situation Room briefing and said, yeah, let the highest officials in our administration fabricate evidence to frame the opposing party candidate Donald Trump. Obama. Biden. Comey. McCabe. Strzok. Page. Rice. Etc.
Knowingly using the faked Clinton campaign ‘Steele Dossier’ hoax, they launched a federal investigation into the Trump presidential campaign that lasted three years after Trump was sworn in as the nation’s 45th President. Arresting and jailing his partners and colleagues. Inventing fake stories for their media enablers. Let’s repeat that. Saint Obama knew there was criminal activity in the process but let his henchmen try to fix an election.

And when the ruse was uncovered no one apologized. No one in authority was fired or jailed. The Pulitzer Prizes awarded to the NT Times and Washington Post for disseminating the DEMs scandal were not rescinded. Nor were they given back by the lying newspapers.
The concerted frauds of the same U.S. DOJ, FBI and State Departments were fed by media and accepted by gullible publics in Canada and America. The fantastical 2020 election results were likewise drummed into the public irrespective of the sudden “appearance” of 27 million new votes during a pandemic.
It was all a fitting preamble to the 2020-2024 Biden senility scandal with Democrats running a man they knew was in full dementia. In the 2020 election Biden was hidden from public view, the better to let media attack Trump for spurious charges launched by partisan DNC attorneys in Georgia, New York and DC. Even then it took the suppression of Hunter Biden’s incriminating laptop just prior to the election to get his father elected.

The dance of denial continued in Biden’s term as he physically and mentally deteriorated before the American public. But inquiries about who was running the government if not Biden were harshly suppressed. Media lackeys noted he was sharp as a tack mentally and in tip-top physical condition when he wasn’t falling down stairs.
It took the stunning 2024 debate debacle with Trump to strip away the lies about Biden’s health, now said to be advanced prostate cancer and Parkinson’s. The media, caught in their own lies about Biden’s condition, offered no apologies and tried to blame Biden’s stutter for the performance.. Right.
These were the two greatest U.S. hoaxes from people who’d cried hoax incessantly. They were hardly the only abuse of public trust. Some of the perpetrators are said to now be under investigation— even as they hand out awards to each other. The media’s credibility is shattered and yet they still blame others. Jaded voters are taking a “we’ll see” approach. But expectations of any change in DC or Ottawa are limited.
As Stephen Taylor posted on X: “Turns out for Liberals, ‘elbows up’ just means ‘noses up’ like it always has.”
Bruce Dowbiggin @dowbboy is the editor of Not The Public Broadcaster A two-time winner of the Gemini Award as Canada’s top television sports broadcaster, his new book Deal With It: The Trades That Stunned The NHL And Changed hockey is now available on Amazon. Inexact Science: The Six Most Compelling Draft Years In NHL History, his previous book with his son Evan, was voted the seventh-best professional hockey book of all time by bookauthority.org . His 2004 book Money Players was voted sixth best on the same list, and is available via brucedowbigginbooks.ca.
Freedom Convoy
Court Orders Bank Freezing Records in Freedom Convoy Case
|
A Canadian court has ordered the release of documents that could shed light on how federal authorities and law enforcement worked together to freeze the bank accounts of a protester involved in the Freedom Convoy.
Both the RCMP and TD Bank are now required to provide records related to Evan Blackman, who took part in the 2022 demonstrations and had his accounts frozen despite not being convicted of any crime at the time.
The Justice Centre for Constitutional Freedoms (JCCF) announced the Ontario Court of Justice ruling. The organization is representing Blackman, whose legal team argues that the actions taken against him amounted to a serious abuse of power.
“The freezing of Mr. Blackman’s bank accounts was an extreme overreach on the part of the police and the federal government,” said his lawyer, Chris Fleury. “These records will hopefully reveal exactly how and why Mr. Blackman’s accounts [were] frozen.”
Blackman was arrested during the mass protests in Ottawa, which drew thousands of Canadians opposed to vaccine mandates and other pandemic-era restrictions.
Although he faced charges of mischief and obstructing police, those charges were dismissed in October due to a lack of evidence. Despite this, prosecutors have appealed, and a trial is set to begin on August 14.
At the height of the protests, TD Bank froze three of Blackman’s accounts following government orders issued under the Emergencies Act. Then-Prime Minister Justin Trudeau had invoked the act to grant his government broad powers to disrupt the protest movement, including the unprecedented use of financial institutions to penalize individuals for their support or participation.
In 2024, a Federal Court Justice ruled that Trudeau’s decision to invoke the act had not been justified.
Blackman’s legal team plans to use the newly released records to demonstrate the extent of government intrusion into personal freedoms.
According to the JCCF, this case may be the first in Canada where a criminal trial includes a Charter challenge over the freezing of personal bank accounts under emergency legislation.
|
-

 International2 days ago
International2 days agoSecret Service suspends six agents nearly a year after Trump assassination attempt
-

 Bruce Dowbiggin1 day ago
Bruce Dowbiggin1 day agoThe Covid 19 Disaster: When Do We Get The Apologies?
-

 Crime23 hours ago
Crime23 hours agoSweeping Boston Indictment Points to Vast Chinese Narco-Smuggling and Illegal Alien Labor Plot via Mexican Border
-

 Alberta1 day ago
Alberta1 day agoAlberta school boards required to meet new standards for school library materials with regard to sexual content
-

 Automotive2 days ago
Automotive2 days agoAmerica’s EV Industry Must Now Compete On A Level Playing Field
-

 Environment23 hours ago
Environment23 hours agoEPA releases report on chemtrails, climate manipulation
-

 Business2 days ago
Business2 days ago‘Experts’ Warned Free Markets Would Ruin Argentina — Looks Like They Were Dead Wrong
-

 Business12 hours ago
Business12 hours agoTrump slaps Brazil with tariffs over social media censorship




