COVID-19
Rep. Paul Gosar introduces bill to end vaccine manufacturer immunity from injury lawsuits
From LifeSiteNews
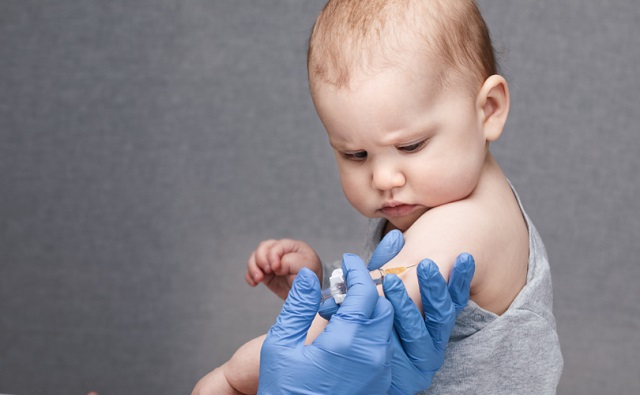
Rep. Paul Gosar’s End the Vaccine Carveout Act would eliminate the general immunity vaccine manufacturers enjoy from vaccine injury civil suits under the federal National Childhood Vaccine Injury Act of 1986.
Republican U.S. Rep. Paul Gosar of Arizona introduced a bill Thursday that would end pharmaceutical companies’ shield against liability for any potential harmful effects of the vaccines they manufacture.
H.R. 9828, the End the Vaccine Carveout Act, would allow individuals to “bring a civil action against a vaccine administrator or manufacturer in a State or Federal court for damages arising from such injury or death,” according to an advance copy of the text provided to LifeSiteNews.
This would eliminate the general immunity vaccine manufacturers enjoy under the federal National Childhood Vaccine Injury Act of 1986, which instead establishes a compensation program for victims. Gosar’s bill allows for civil actions to be pursued regardless of whether a victim has filed a petition with the program, although ultimately receiving an award from one would invalidate a petition to the other.
“Government bureaucrats and scientists responsible for approving vaccines are in bed with Big Pharma, often owning pharmaceutical stocks, serving as consultants and receiving lucrative contracts from pharmaceutical companies that pressure them to produce favorable results which is in direct violation of federal law,” Gosar said in a press release. “Big Pharma doesn’t deserve a get-out-of-jail-free card for injuries caused by their harmful vaccines.”
The question of vaccine safety has become more mainstream in recent years due to the controversy surrounding the COVID-19 vaccines, which were developed and reviewed in a fraction of the time vaccines usually take under the Trump administration’s Operation Warp Speed initiative.
A large body of evidence identifies significant risks to the COVID vaccines. Among it, the federal Vaccine Adverse Event Reporting System (VAERS) reports 37,910 deaths, 217,931 hospitalizations, 21,917 heart attacks, and 28,602 myocarditis and pericarditis cases as of September 6, among other ailments. U.S. Centers for Disease Control and Prevention (CDC) researchers have recognized a “high verification rate of reports of myocarditis to VAERS after mRNA-based COVID-19 vaccination,” leading to the conclusion that “under-reporting is more likely” than over-reporting.
An analysis of 99 million people across eight countries published February in the journal Vaccine “observed significantly higher risks of myocarditis following the first, second and third doses” of mRNA-based COVID vaccines, as well as signs of increased risk of “pericarditis, Guillain-Barré syndrome, and cerebral venous sinus thrombosis,” and other “potential safety signals that require further investigation.” In April, the CDC was forced to release by court order 780,000 previously undisclosed reports of serious adverse reactions, and a study out of Japan found “statistically significant increases” in cancer deaths after third doses of mRNA-based COVID-19 vaccines, and offered several theories for a causal link.
In Florida, an ongoing grand jury investigation into the vaccines’ manufacturers is slated to release a highly anticipated report on the shots, and a lawsuit by the state of Kansas has been filed accusing Pfizer of misrepresentation for calling the shots “safe and effective.”
Yet so far, Big Pharma has largely escaped accountability thanks to both the aforementioned 1986 law and the federal Public Readiness and Emergency Preparedness (PREP) Act of 2005.
According to the Congressional Research Service (CRS), the PREP Act empowers the federal government to “limit legal liability for losses relating to the administration of medical countermeasures such as diagnostics, treatments, and vaccines.” Near the beginning of the 2020 COVID-19 outbreak, the Trump administration invoked the Act in declaring the virus a “public health emergency.”
Under this “sweeping” immunity, CRS explained, the federal government, state governments, “manufacturers and distributors of covered countermeasures,” and licensed or otherwise-authorized health professionals distributing those countermeasures are shielded from “all claims of loss” stemming from those countermeasures, with the exception of “death or serious physical injury” brought about through “willful misconduct,” a standard that, among other hurdles, requires the offender to have acted “intentionally to achieve a wrongful purpose.”
Many hope that by going after Big Pharma for misrepresentations surrounding their products rather than the products themselves, efforts like the Kansas suit can circumvent that hurdle to impose consequences on those responsible for the shots.
COVID-19
Judge denies Canadian gov’t request to take away Freedom Convoy leader’s truck

From LifeSiteNews
A judge ruled that the Ontario Court of Justice is already ‘satisfied’ with Chris Barber’s sentence and taking away his very livelihood would be ‘disproportionate.’
A Canadian judge has dismissed a demand from Canadian government lawyers to seize Freedom Convoy leader Chris Barber’s “Big Red” semi-truck.
On Friday, Ontario Court of Justice Judge Heather Perkins-McVey denied the Crown’s application seeking to forfeit Barber’s truck.
She ruled that the court is already “satisfied” with Barber’s sentence and taking away his very livelihood would be “disproportionate.”
“This truck is my livelihood,” said Barber in a press release sent to LifeSiteNews.
“Trying to permanently seize it for peacefully protesting was wrong, and I’m relieved the court refused to allow that to happen,” he added.
Criminal defense lawyer Marwa Racha Younes was welcoming of the ruling as well, stating, “We find it was the right decision in the circumstances and are happy with the outcome.”
John Carpay, president of the Justice Centre for Constitutional Freedoms (JCCF), said the decision is “good news for all Canadians who cherish their Charter freedom to assemble peacefully.”
READ: Freedom Convoy protester appeals after judge dismissed challenge to frozen bank accounts
“Asset forfeiture is an extraordinary power, and it must not be used to punish Canadians for participating in peaceful protest,” he added in the press release.
As reported recently by LifeSiteNews, the Canadian government claimed that Barber’s truck is an “offence-related property” relating to his involvement in the 2022 protests against Canada’s COVID mandates.
At this time, the court ruling ends any forfeiture proceedings for the time being, however Barber will continue to try and appeal his criminal conviction and house arrest sentence.
Barber’s truck, a 2004 Kenworth long-haul he uses for business, was a focal point in the 2022 protests. He drove it to Ottawa, where it was parked for an extended period of time, but he complied when officials asked him to move it.
On October 7, 2025, after a long trial, Ontario Court Justice Perkins-McVey sentenced Barber and Tamara Lich, the other Freedom Convoy leader, to 18 months’ house arrest. They had been declared guilty of mischief for their roles as leaders of the 2022 protest against COVID mandates, and as social media influencers.
Lich and Barber have filed appeals of their own against their house arrest sentences, arguing that the trial judge did not correctly apply the law on their mischief charges.
Government lawyers for the Crown have filed an appeal of the acquittals of Lich and Barber on intimidation charges.
The pair’s convictions came after a nearly two-year trial despite the nonviolent nature of the popular movement.
COVID-19
Freedom Convoy protester appeals after judge dismissed challenge to frozen bank accounts

From LifeSiteNews
Protestor Evan Blackman’s legal team argues Trudeau’s Emergencies Act-based bank account freezes were punitive state action tied directly to protest participation.
A Freedom Convoy protester whose bank accounts were frozen by the Canadian government says a judge erred after his ruling did not consider the fact that the funds were frozen under the Emergencies Act, as grounds for a stay of proceedings.
In a press release sent out earlier this week, the Justice Centre for Constitutional Freedoms (JCCF) said that Freedom Convoy protestor Evan Blackman will challenge a court ruling in his criminal case via an appeal with the Ontario Superior Court of Justice.
“This case raises serious questions about how peaceful protest is treated in Canada and about the lasting consequences of the federal government’s unlawful use of the Emergencies Act,” noted constitutional lawyer Chris Fleury. “The freezing of protestors’ bank accounts was part of a coordinated effort to suppress dissent, and courts ought to be willing to scrutinize that conduct.”
Blackman was arrested on February 18, 2022, during the police crackdown on Freedom Convoy protests against COVID restrictions, which was authorized by the Emergencies Act (EA). The EA was put in place by former Prime Minister Justin Trudeau’s Liberal government, which claimed the protests were violent, despite no evidence that this was the case.
Blackman’s three bank accounts with TD Bank were frozen due to his participation in the Freedom Convoy, following a directive ordered by Trudeau.
As reported by LifeSiteNews, in November of this year, Blackman was convicted at his retrial even though he had been acquitted at his original trial. In 2023, Blackman’s “mischief” and “obstructing police” charges were dismissed by a judge due to lack of evidence and the “poor memory of a cop regarding key details of the alleged criminal offences.”
His retrial resulted in Blackman getting a conditional discharge along with 12 months’ probation and 122 hours of community service, along with a $200 victim fine surcharge.
After this, Blackman’s application for a stay of proceedings was dismissed by the court. He had hoped to have his stay of proceedings, under section 24(1) of the Charter of Rights and Freedoms, allowed. However, the judge ruled that the freezing of his bank accounts was legally not related to his arrest, and because of this, the stay of proceedings lacked standing.
The JCCF disagreed with this ruling, noting, it “stands in contrast to a Federal Court decision finding that the government’s invocation of the Emergencies Act was unreasonable and violated Canadians’ Charter rights, including those targeted by the financial measures used against Freedom Convoy protestors.”
As of press time, a hearing date has not been scheduled.
In 2024, Federal Court Justice Richard Mosley ruled that Trudeau was “not justified” in invoking the Emergencies Act.
In early 2022, the Freedom Convoy saw thousands of Canadians from coast to coast come to Ottawa to demand an end to COVID mandates in all forms. Despite the peaceful nature of the protest, Trudeau’s federal government enacted the EA in mid-February.
After the protesters were cleared out, which was achieved through the freezing of bank accounts of those involved without a court order as well as the physical removal and arrest of demonstrators, Trudeau revoked the EA on February 23, 2022.
-

 Business1 day ago
Business1 day agoLargest fraud in US history? Independent Journalist visits numerous daycare centres with no children, revealing massive scam
-

 Business1 day ago
Business1 day ago“Magnitude cannot be overstated”: Minnesota aid scam may reach $9 billion
-

 Business2 days ago
Business2 days agoSocialism vs. Capitalism
-

 Censorship Industrial Complex22 hours ago
Censorship Industrial Complex22 hours agoUS Under Secretary of State Slams UK and EU Over Online Speech Regulation, Announces Release of Files on Past Censorship Efforts
-

 Energy2 days ago
Energy2 days agoCanada’s debate on energy levelled up in 2025
-

 Daily Caller2 days ago
Daily Caller2 days agoIs Ukraine Peace Deal Doomed Before Zelenskyy And Trump Even Meet At Mar-A-Lago?
-

 Business23 mins ago
Business23 mins agoWhat Do Loyalty Rewards Programs Cost Us?
-

 Haultain Research33 mins ago
Haultain Research33 mins agoSweden Fixed What Canada Won’t Even Name



