COVID-19
Ontario doctor punished for questioning COVID response plans appeal to Supreme Court

Ontario pediatrician Dr. Kulvinder Kaur Gill
From LifeSiteNews
Elon Musk has said he would help Dr. Kulvinder Kaur Gill financially in her fight against the College of Physicians and Surgeons of Ontario.
Ontario pediatrician Dr. Kulvinder Kaur Gill, who is embroiled in a legal battle with a medical regulator for her anti-COVID jab and mandate views on social media, is looking to take her case to Canada’s Supreme Court with financial help from Elon Musk and a leading freedom-fighting lawyer.
Libertas Law, which is representing Gill, said in a press release sent to LifeSiteNews on Monday the canceled doctor “filed an application for leave to appeal to the Supreme Court of Canada” her case against the College of Physicians and Surgeons of Ontario (CPSO).
“The growing overreach of regulators into monitoring the speech of professionals on social media has become a matter of national concern to the public, which loses the benefit of hearing a variety of opinions when professionals’ speech is chilled out of fear of punishment,” Libertas Law attorney Lisa Bildy said. “We hope that the Supreme Court of Canada will use Dr. Gill’s case to restore the historic role of the courts as guardians of the constitution.”
The application follows Gill’s unsuccessful judicial review of the “cautions-in-person ordered against her in 2021” by a CPSO committee concerning her Twitter comments in August 2020 that criticized multiple levels of governments COVID mandates and policies.
The orders against Gill were made despite her “providing the College with ample evidence in 2020 to support her position against catastrophic lockdowns,” Libertas Law noted.
Musk, the billionaire Tesla and X owner, pledged in March to back Gill financially.
The application to Canada’s highest court comes after her application for leave to appeal to the Ontario Court of Appeal (ONCA) “was denied” on October 3.
“The infringement of Dr. Gill’s freedom of expression and conscience, guaranteed under the Charter of Rights and Freedoms, was barely mentioned by the committee when it issued the orders for cautions in-person (which Dr. Gill has not yet received),” Libertas stated in its press release.
Libertas noted that a brief comment about the committee having “no interest in shutting down free speech” was made “before proceeding to do exactly that.”
According to Libertas, the CPSO had placed on its website in 2020 a warning to doctors to provide “an opinion that does not align with information coming from public health or government.”
“Yet the Divisional Court declined to quash the orders, finding that the committee was sufficiently alert to the Charter infringement of Dr. Gill’s speech, such that its decisions were within the range of reasonable outcomes,” the legal firm said.
Last May, LifeSiteNews reported that Gill had vowed to fight with appeals with the help of her Musk-backed legal team after she lost a court battle.
One of Gill’s “controversial” posts she made in 2020 read, “If you have not yet figured out that we don’t need a vaccine, you are not paying attention. #FactsNotFear.”
The Divisional Court decision against Gill dated May 7 concluded, “When the College chose to draw the line at those tweets which it found contained misinformation, it did so in a way which reasonably balanced Dr. Gill’s free speech rights with her professional responsibilities.”
“In other words, its response was proportionate,” the ruling stated.
In Monday’s press release, Libertas Law noted that due to an unrelated recent court ruling relating to Charter Rights, Gill will argue the same reasonings to fight her censorship in her appeal to the Supreme Court.
Canceled doc’s legal battles against medical regulator ongoing for months
Gill’s court challenge against the CPSO began earlier this year, with Bildy writing at the time that the “decisions were neither reasonable nor justified and they failed to engage with the central issues for which Dr. Gill was being cautioned.”
She argued that Gill had a “reasonable scientific basis” for her posts, noting that the previous decision made against Gill targeted her for opposing the mainstream COVID narrative.
Gill is a specialist practicing in the Toronto area and has extensive experience and training in “pediatrics, and allergy and clinical immunology, including scientific research in microbiology, virology and vaccinology.”
Last September, disciplinary proceedings against her were withdrawn by the CPSO. However, Gill was ordered last year to pay $1 million in legal costs after her libel suit was struck down.
The CPSO began disciplinary investigations against Gill in August 2020.
COVID vaccine mandates, which came from provincial governments with the support of the federal government, split Canadian society. The mRNA shots have been linked to a multitude of negative and often severe side effects in children.
In an interview with LifeSiteNews at its annual general meeting in July 2023 near Toronto, canceled doctors Mary O’Connor, Mark Trozzi, Chris Shoemaker, and Byram Bridle were asked to state their messages to the medical community regarding how they have had to fight censure because they have opinions contrary to the COVID mainstream narrative.
COVID-19
Nearly Half of “COVID-19 Deaths” Were Not Due to COVID-19 – Scientific Reports Journal


 Nicolas Hulscher, MPH
Nicolas Hulscher, MPH
45.3% of “COVID-19 deaths” in Greece had no symptoms — exposing the coordinated PSYOP deployed to maximize fear and enforce mass compliance with draconian control measures.
The study titled “Deaths “due to” COVID-19 and deaths “with” COVID-19 during the Omicron variant surge, among hospitalized patients in seven tertiary-care hospitals, Athens, Greece” was just published in the journal Scientific Reports:
Abstract
In Greek hospitals, all deaths with a positive SARS-CoV-2 test are counted as COVID-19 deaths. Our aim was to investigate whether COVID-19 was the primary cause of death, a contributing cause of death or not-related to death amongst patients who died in hospitals during the Omicron surge and were registered as COVID-19 deaths. Additionally, we aimed to analyze the factors associated with the classification of these deaths. We retrospectively re-viewed all in-hospital deaths, that were reported as COVID-19 deaths, in 7 hospitals, serving Athens, Greece, from January 1, 2022, until August 31, 2022. We retrieved clinical and laboratory data from patient records. Each death reported as COVID-19 death was characterized as: (A) death “due to” COVID-19, or (B) death “with” COVID-19. We reviewed 530 in-hospital deaths, classified as COVID-19 deaths (52.4% males; mean age 81.7 ± 11.1 years). We categorized 290 (54.7%) deaths as attributable or related to COVID-19 and in 240 (45.3%) deaths unrelated to COVID-19. In multivariable analysis The two groups differed significantly in age (83.6 ± 9.8 vs. 79.9 ± 11.8, p = 0.016), immunosuppression history (11% vs. 18.8%, p = 0.027), history of liver disease (1.4% vs. 8.4%, p = 0.047) and the presence of COVID-19 symptoms (p < 0.001). Hospital stay was greater in persons dying from non-COVID-19 related causes. Among 530 in-hospital deaths, registered as COVID-19 deaths, in seven hospitals in Athens during the Omicron wave, 240 (45.28%) were reassessed as not directly attributable to COVID-19. Accuracy in defining the cause of death during the COVID-19 pandemic is of paramount importance for surveillance and intervention purposes.
Key Findings:
Massive Overcounting of COVID-19 Deaths
- Out of 530 hospital deaths registered as COVID-19 deaths, only 290 (54.7%) were actually caused by COVID-19.
- 240 deaths (45.3%) were found to be completely unrelated to COVID-19 — patients died with a positive PCR test, but showed no symptoms, required no COVID-specific treatment, and died of clearly unrelated causes.
Death Certificate Inaccuracy
- Of the 204 certificates listing COVID-19 as the direct cause of death, only 132 (64.7%) were confirmed as such after clinical review.
- Of the 324 certificates listing COVID-19 as a contributing factor, only 86 (26.5%) were found to be truly related.
Hospital-Acquired Infections Misclassified
- Patients infected during hospitalization were significantly more likely to be misclassified as COVID-19 deaths (OR: 2.3, p = 0.001).
Younger Age and Severe Comorbidities Associated with Misclassification
- Patients who died “with” COVID-19 were younger, more likely to be immunosuppressed, have end-stage liver disease, or be admitted for other causes.
Symptoms and Treatments Differed Sharply
Patients who died “due to” COVID-19 were more likely to:
- Exhibit classic symptoms: hypoxia (44.1%), shortness of breath, fever, and cough
- Require oxygen support (93.4% vs. 66.9%) and receive COVID-specific therapies:
- Remdesivir (5-day course: 61.9% vs. 35.2%)
- Dexamethasone (81.7% vs. 40.7%)
Study Strengths
This study went far beyond death certificate coding, implementing a rigorous, multi-source clinical audit:
- Full medical chart reviews: Included physician notes, lab data, imaging, and treatment records.
- Attending physician interviews: Structured questionnaires captured real-time clinical insights from those who treated the patients.
- Dual independent expert assessments: Two experienced infectious disease specialists (each with >2,500 COVID cases) reviewed each case independently for classification accuracy.
This study found that nearly half of all registered COVID-19 deaths during the Omicron wave in Greece were misclassified, with no clinical evidence linking them to COVID-19 as the true cause. Given that similar death coding practices were employed across Western nations, it is reasonable to conclude that COVID-19 death counts were artificially inflated to a comparable degree elsewhere.
This drastic inflation of death counts aligns with what many now understand to be a coordinated psychological operation (PSYOP)—designed to instill fear and maximize compliance with draconian pandemic measures such as lockdowns, mask mandates, and mass mRNA injection campaigns.
It is this weaponization of fear that has prompted criminal referrals in seven U.S. states, triggering active criminal investigations into top COVID-19 officials for terrorism, murder and racketeering:
BREAKING – The Pandemic Justice Phase Begins as Criminal Investigations Commence |
||||||
|
||||||
|
By Nicolas Hulscher, MPH
|
||||||
|
Epidemiologist and Foundation Administrator, McCullough Foundation
Please consider following both the McCullough Foundation and my personal account on X (formerly Twitter) for further content.
2025 Federal Election
Before the Vote: Ask Who’s Defending Our Health

From the World Council for Health Canada
The health of Canadians has been compromised by government-mandated COVID-19 injections. The upcoming federal election is an opportunity to demand change and accountability. As you decide which candidate or party is most committed to defending the health of yourself and your family, please consider the following:
The Injections Were Never What They Claimed
The Canadian government successfully mandated the COVID-19 injections by labeling them “safe and effective vaccines.” These products are still being promoted and administered across the country. However, the truth is:
- They are not vaccines: Click Here
- They are not safe: Click Here
- They do not prevent infection or transmission.
- Evidence shows they increase the risk of COVID-19 disease and death: Click Here
These Products Contain Multiple Mechanisms of Harm
- They cause injury through multiple biological mechanisms: Click Here
- They have surpassed all vaccines in recorded history—for all infections, for all of the past thirty years combined—in causing deaths and injuries: Click Here
- They are chemically contaminated and adulterated with DNA: Click Here
- In Pfizer’s case, fraud is evident: the DNA contamination includes genetic engineering tools derived from the SV40 virus, associated with cancer risks: Click Here
This Election, We Must Demand Accountability
Insist that to have your vote, candidates must:
- Denounce the COVID-19 “vaccines.”
- Support a full halt to their manufacturing and administration.
- Uphold informed consent, scientific integrity, and bodily autonomy.
Your voice is important. Use it to reject censorship, harm, and medical coercion.
-

 2025 Federal Election13 hours ago
2025 Federal Election13 hours agoBREAKING: THE FEDERAL BRIEF THAT SHOULD SINK CARNEY
-

 2025 Federal Election14 hours ago
2025 Federal Election14 hours agoCHINESE ELECTION THREAT WARNING: Conservative Candidate Joe Tay Paused Public Campaign
-
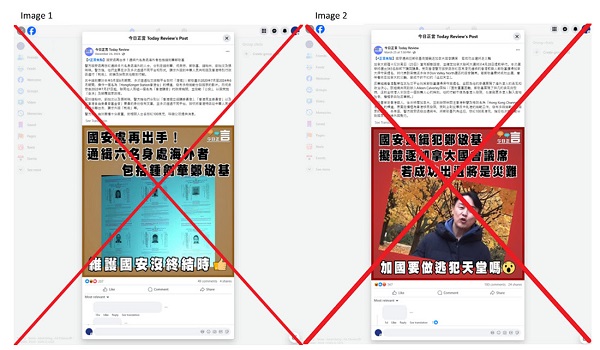
 2025 Federal Election1 day ago
2025 Federal Election1 day agoOttawa Confirms China interfering with 2025 federal election: Beijing Seeks to Block Joe Tay’s Election
-

 2025 Federal Election24 hours ago
2025 Federal Election24 hours agoReal Homes vs. Modular Shoeboxes: The Housing Battle Between Poilievre and Carney
-

 2025 Federal Election2 days ago
2025 Federal Election2 days agoCarney’s budget means more debt than Trudeau’s
-

 International2 days ago
International2 days agoPope Francis has died aged 88
-

 Business2 days ago
Business2 days agoCanada Urgently Needs A Watchdog For Government Waste
-

 2025 Federal Election24 hours ago
2025 Federal Election24 hours agoHow Canada’s Mainstream Media Lost the Public Trust



