Health
Canadian dentists desperate for details on federal dental care plan
News release from Canada’s Provincial and Territorial Dental Associations
Canadian dentists to MPs: We need answers about the Canadian Dental Care Plan
Lack of consultation with provincial and territorial dental associations is worrying
There are only two months left before the Canadian Dental Care Plan (CDCP) becomes available to many more Canadians. Yet more than 25,000 dentists nationwide are in the dark about how the Government of Canada will safeguard access to dental care.
In a letter sent to Members of Parliament (MPs) this week, the presidents of provincial and territorial dental associations across the country asked how the government will:
- Safeguard employer-provided dental plans that two-thirds of Canadians currently have access to?
- Ensure that a strong federal program can be coordinated with existing provincial programs?
- Protect patient choice and maintain the patient-provider relationship?
- Ensure minimal, efficient administration that promotes timely access to care?
- Respect the costs of delivering dental care to maximize provider participation?
- Increase the number of dental assistants and dental hygienists to meet the demands of the CDCP?
Dentists want to champion a CDCP that will respect patients, providers, and taxpayers. The provincial and territorial dental associations are concerned that the CDCP has been compromised by a lack of meaningful consultation with dentists – who will be expected to deliver on the government’s promises.
The CDCP is currently in final planning stages, with a potential rollout in 2024 that will attempt to increase access to uninsured Canadians under 18, people with disabilities, and seniors who have an annual family income of less than $90,000. Dentists believe all Canadians need access to dental care. If not done properly, two-thirds of Canadians who have great employer-provided dental plans could lose their coverage and be forced into a worse plan. Costs would then skyrocket, which means the $13 billion over five years the government set aside would not be enough to sustain the plan.
Let’s take the time to get it right. We can increase access to dental care right now through an expansion of the interim measure already in place – the Canada Dental Benefit. This establishes a fixed dollar amount that a patient can use to be reimbursed for dental-related expenses.
Facts:
- Canada’s provincial and territorial dental associations represent more than 25,000 licensed dentists working in more than 16,000 offices. They treat more than 30 million Canadians every year and employ at least 50,0001 oral health care workers.
- Over 60 per cent of Canadians have a dentist they visit on a regular basis.2
- A recent survey commissioned by Health Canada found that nearly nine out 10 Canadians are satisfied with the Canada Dental Benefit.3
Quotes:
“To succeed, this plan needs to work for both patients and providers, and to work in each province. What we are recommending is based on decades of experience and caring for the oral health needs of the more than 30 million people that come into our dental offices across the country every year.” — Dr. Bruce Yaholnitsky, President, Alberta Dental Association
“Poorly designed programs do not improve access to care, and they leave the most vulnerable people in society behind. This is an historic opportunity, but only if the government gets it right. Dentists have the expertise, experience, and skills to know what it takes to ensure good oral and overall health.” — Dr. Rob Wolanski, President, British Columbia Dental Association
“As dentists we are excited to be a part of this Canadian dental care program, but there are key critical issues that need to be included for this program to be successful.” — Dr. Scott Leckie, President, Manitoba Dental Association
“New Brunswick dentists are already extremely busy with the recent spike in population and the backlog in demand for services related to Covid-19. This program was intended to provide dental care to the 35 per cent of Canadians who are uninsured. It needs to be easy to understand and to administer, and to be fair to all parties, including patients, dental care providers and taxpayers. Canadians need to know what benefits are being provided and which are not, before they arrive at the dental clinic.” — Dr. Joanah Campbell, President, New Brunswick Dental Society
“The new program must be sustainable in terms of funding, and easy to understand and access. It has to be patient-centred and work for everyone.” — Dr. Shane Roberts, President, Newfoundland & Labrador Dental Association
“While the CDCP has the potential to improve the lives of many Canadians, this can only be achieved if it’s done right. To ensure the greatest possible outcome, we must consider all of the moving pieces and take a patient-centred approach.” — Dr. Juli Waterbury, President, Nova Scotia Dental Association
“The CDCP could be a game-changer for Canadians’ access to dental care. But we have one chance to get it right. Here in Ontario, we have seen that dental care programs developed without the input of dentists are doomed to fail. Just look at the Ontario Seniors Dental Care Program, where waiting lists are up to two years long in some areas, and some patients have to travel ridiculously long distances to receive treatment.” — Dr. Brock Nicolucci, President, Ontario Dental Association
“This new program has the potential to improve access to care for many Canadians. It must be sustainable, patient-centred, and easy to access for patients. A poorly designed program will not improve access to care which is something we would like to avoid. We want this to work for Canadians.” — Dr. Derek Thiessen, President, College of Dental Surgeons of Saskatchewan
Health
US podcaster Glenn Beck extends a lifeline to a Saskatchewan woman waiting for MAiD

From LifeSiteNews
Jolene Van Alstine was approved for euthanasia after tiring of waiting years for surgery in Canada
A Canadian woman is looking to die by state-sanctioned euthanasia because she has had to endure long wait times to get what she considers to be proper care for a rare parathyroid disease.
The woman is Jolene Van Alstine, whose condition, normocalcemic primary hyperparathyroidism (nPHPT), causes her to experience vomiting, nausea, and bone pain.
As noted in a recent CBC report, Van Alstine claims she is not able to get proper surgery to remove her parathyroid in her home province of Saskatchewan, as there are no surgeons in that province who can perform that type of surgery.
She has said her “friends have stopped visiting me” and she is “isolated” and living “alone lying on the couch for eight years, sick and curled up in a ball, pushing for the day to end.”
“I go to bed at six at night because I can’t stand to be awake anymore,” she said.
As a result of her frustrations with the healthcare system, Van Alstine applied for Canada’s Medical Assistance in Dying (MAiD). She was approved for the procedure on January 7, 2026.
Saskatchewan Health Minister Jeremy Cockrill met with Van Alstine last month to try to see if he could help her, but what they talked about remains confidential.
“The Government of Saskatchewan expresses its sincere sympathy for all patients who are suffering with a difficult health diagnosis,” the government said.
As reported by LifeSiteNews, over 23,000 Canadians have died while on wait lists for medical care as Prime Minister Mark Carney’s Liberal government is focused on euthanasia expansions.
A new Euthanasia Prevention Coalition report revealed that Canada has euthanized 90,000 people since 2016, the year it was legalized.
Americans offering Jolene surgery help now say they have made contact with her
Van Alstine’s story has gone viral on the social media platform X, catching the attention of well-known American personalities, some who have claimed they can help her.
“If there is any surgeon in America who can do this, I’ll pay for this patient to come down here for treatment,” Glenn Beck wrote Tuesday on X.
“THIS is the reality of ‘compassionate’ progressive healthcare. Canada must END this insanity and Americans can NEVER let it spread here.”
According to Beck in a subsequent X post, he has had “surgeons who emailed us standing by to help her.”
“We are in contact with Jolene and her husband! Please continue to pray for her health,” he wrote on X.
“Will update more soon.”
As reported by LifeSiteNews recently, a Conservative MP’s private member’s bill that, if passed, would ban euthanasia for people with mental illness received the full support of the Euthanasia Prevention Coalition.
Lobby groups have pushed for MAiD to be expanded to minors.
Desiring to expand the procedure to even more Canadians, former Prime Minister Justin Trudeau’s government sought to expand from just the chronically and terminally ill to those suffering solely from mental illness. The current Liberal government appears to want to continue with the MAiD regime.
However, in February, after pushback from pro-life, medical, and mental health groups as well as most of Canada’s provinces, the federal government delayed the mental illness expansion until 2027.
Health
The Data That Doesn’t Exist

ACIP voted to un-recommend the Hep B birth dose, but here’s the problem: they still can’t weigh the other side of the ledger
Sunday, something happened that has never happened in the history of American public health: ACIP voted 8-3 to un-recommend the universal birth dose of hepatitis B for babies born to mothers who test negative for the virus. After 34 years of jabbing every American newborn within hours of taking their first breath—regardless of whether their mother had hepatitis B—the committee finally acknowledged what 25 European countries figured out decades ago: it doesn’t make sense.
But watching this vote unfold, I couldn’t help but notice the absurdity of the debate itself. Committee members who opposed the change kept saying variations of the same thing: “We’ve heard ‘do no harm’ as a moral imperative. We are doing harm by changing this wording.” Another said “no rational science has been presented” to support the change.
How to End the Autism Epidemic is a reader-supported publication.
To receive new posts and support my work, consider becoming a free or paid subscriber.
And therein lies the fundamental problem with ACIP—and with the entire vaccine regulatory apparatus in America. They literally cannot weigh risk versus benefit because they only have data on one side of the scale.
The Missing Side of the Ledger
When ACIP debates adding or removing a vaccine from the schedule, they can produce endless data on disease incidence. They can show you charts demonstrating how hepatitis B cases in infants dropped from thousands to single digits after 1991. They can model projected infections if vaccination rates decline. They have this data at their fingertips because tracking infectious disease is something our public health apparatus actually does.
But ask them to produce equivalent data on vaccine injury, and you’ll get silence. Not “the data shows injuries are rare.” Not “here’s our comprehensive tracking of adverse events.” Just… nothing. A void where information should be.
This is not an accident. This is by design.
The safety trials for Engerix-B and Recombivax HB—the two hepatitis B vaccines given to American newborns—monitored adverse events for four to five days after injection. That’s it. If your baby developed seizures on day six, or regressed into autism over the following months, or developed autoimmune disease in the following year—none of that would appear in the pre-licensure safety data.
And the post-market surveillance? VAERS is a voluntary reporting system that the CDC itself acknowledges captures only a tiny fraction of adverse events. A Harvard-funded study found it captures perhaps 1% of actual vaccine injuries. Vaccine court has paid out over $5 billion in claims while simultaneously being structured to make filing nearly impossible for average families.
So when Dr. Cody Meissner voted against removing the Hep B birth dose and said he saw “clear evidence of the benefits” but “not the harms,” he was accidentally revealing the entire rotten structure. Of course he doesn’t see the harms. Nobody is systematically looking for them.
The Invisibility of Vaccine Injury
Here’s what most people don’t understand about vaccine injury: it’s nothing like a gunshot wound.
If you shoot someone, the cause is obvious. There’s a bullet, a wound, blood, a clear mechanism of action visible to any observer. Even a medical examiner who’s never seen the victim before can determine cause of death.
Vaccine injury doesn’t work that way. When aluminum nanoparticles from a vaccine cross the blood-brain barrier via macrophages, when they lodge in brain tissue and trigger chronic neuroinflammation, when a child slowly regresses over weeks or months—there’s no bullet. There’s no smoking gun. There’s just a before and an after, and a desperate parent trying to explain to doctors that something changed.
This invisibility is the vaccine program’s greatest protection. Because the injury mechanism is complex and delayed, because it doesn’t leave an obvious wound, because it requires actually looking to find—and because no one in authority is looking—the injuries simply don’t exist in the official record.
I watched my own son Jamie regress after his vaccines. A healthy, developing toddler who lost his words, stopped making eye contact, and retreated into a world we couldn’t reach. My wife and I know what happened. Thousands of other parents know the same thing happened to their children. But because this type of injury doesn’t show up on a simple blood test, because there’s no autopsy finding that says “vaccine-induced encephalopathy,” ACIP members can sit in a room and say with straight faces that they don’t see evidence of harm.
They’re not lying. They literally can’t see it. Because no one is measuring it.
The Chicken Pox Conundrum
Here’s an example that illustrates the insanity of our current approach.
The varicella (chicken pox) vaccine was added to the schedule in 1995. It definitely reduces chicken pox cases. The data is clear on that front. Mission accomplished, right?
But what about the other side of the ledger?
Emerging research suggests that wild chicken pox infection provides some protective effect against brain cancers—particularly glioma, the most common type of primary brain tumor. Multiple studies have found that people who had chicken pox as children have significantly lower rates of brain cancer later in life. The hypothesis is that the immune response to wild varicella provides lasting immunological benefits that extend far beyond preventing itchy spots.
Meanwhile, the vaccine itself has been associated with increased rates of autoimmune conditions. Studies have linked varicella vaccination to higher rates of herpes zoster (shingles) outbreaks in younger age groups, to autoimmune disorders, to various adverse events that weren’t captured in the original short-term safety trials.
So what’s the true risk-benefit of the chicken pox vaccine? Does preventing a week of itchy discomfort in childhood justify potentially increased rates of brain cancer and autoimmune disease later in life?
ACIP can’t answer this question. They literally don’t have the data. They can show you chicken pox cases going down. They cannot show you a comprehensive analysis of long-term neurological and immunological outcomes in vaccinated versus unvaccinated populations, because that study has never been done.
And so they keep recommending the vaccine based on the only data they have—the disease prevention data—while remaining willfully blind to consequences they’ve never bothered to measure.
The ACIP Paradox
Sunday’s vote was historic, but it also revealed the fundamental paradox of vaccine regulation in America.
The committee members who voted to remove the universal Hep B birth dose recommendation did so largely based on comparative evidence from Europe, parental concerns, and the basic logic that vaccinating a 12-hour-old baby for a sexually transmitted disease their mother doesn’t have makes no medical sense. They were right to do so.
But the committee members who voted against the change weren’t wrong either, from their perspective. They looked at the only data they have—disease prevention data—and concluded that removing the recommendation could lead to more hepatitis B cases. And within their limited framework, they’re correct.
The problem is the framework itself.
True risk-benefit analysis requires data on both risks AND benefits. ACIP has comprehensive data on benefits (disease prevention) and virtually no data on risks (vaccine injury). So every decision they make is fundamentally flawed from the start.
When Dr. Joseph Hibbeln complained that “no rational science has been presented” to support changing the recommendations, he was inadvertently indicting the entire system. Of course no comprehensive vaccine injury data was presented—such data doesn’t exist because no one has been willing to collect it.
This is like asking someone to make an informed financial decision while only showing them potential profits and hiding all possible losses. Of course the decision will be skewed. Of course you’ll end up with a bloated portfolio of high-risk investments that look great on paper.
The Real Reform
If RFK Jr. and the new HHS leadership want to actually fix the vaccine program, they need to understand that removing individual vaccines or making them “optional” is just rearranging deck chairs on the Titanic.
The real reform is creating the data infrastructure that should have existed from the beginning.
We need a comprehensive, long-term, vaccinated-versus-unvaccinated health outcomes study. Not a five-day safety trial. A multi-decade tracking of neurological, immunological, and developmental outcomes across populations with varying vaccination status. Florida just eliminated all vaccine mandates—that state alone could provide the data we need within ten years if someone had the courage to actually collect it.
We need a vaccine injury surveillance system that actually captures adverse events. Not a voluntary reporting system that misses 99% of injuries. An active surveillance system with trained clinicians looking for the kinds of delayed, complex injuries that vaccines actually cause.
We need accountability for manufacturers. The 1986 National Childhood Vaccine Injury Act removed all liability from vaccine makers—and predictably, the vaccine schedule exploded afterward while safety research stagnated. Why would any company invest in safety when they can’t be sued for injuries?
Without this data, every ACIP meeting will be the same performance we watched this week: members confidently citing disease prevention data while admitting they can’t see evidence of harm—not because harm doesn’t exist, but because no one is looking for it.
What Comes Next
Sunday’s vote was a crack in the wall. For the first time, an American regulatory body acknowledged that perhaps vaccinating every newborn within hours of birth for a disease primarily transmitted through sex and IV drug use doesn’t make sense when the mother has already tested negative.
But the forces of institutional inertia are already mobilizing. The American Academy of Pediatrics is “disappointed.” The American Medical Association is calling for the CDC to reject the recommendation. The pharmaceutical industry—which collects over $225 million annually from Hep B birth doses alone—will fight to restore the universal recommendation.
They will cite the same data they always cite: disease prevention data. Cases prevented. Infections avoided. Lives saved—theoretically.
They will not cite vaccine injury data, because that data doesn’t exist in any comprehensive form. They will not present long-term health outcomes in vaccinated versus unvaccinated children, because those studies have been actively avoided for decades. They will not acknowledge the thousands of families who have watched their children regress after vaccination, because those injuries aren’t captured in any official database.
And this is why ACIP will always be hamstrung. Until we build the data infrastructure to actually measure vaccine injury—to put real numbers on the other side of the ledger—every vaccine decision will be based on incomplete information. Every “risk-benefit analysis” will be a fraud, because we’re only measuring half the equation.
The hepatitis B birth dose vote was a small victory. But the larger battle—for actual science, for complete data, for true informed consent—that battle is just beginning.
And until we win it, ACIP will continue making decisions in the dark, confidently citing evidence of benefits while remaining deliberately blind to the harms they’ve never bothered to measure.
About the author
J.B. Handley is the proud father of a child with Autism. He spent his career in the private equity industry and received his undergraduate degree with honors from Stanford University. His first book, How to End the Autism Epidemic, was published in September 2018. The book has sold more than 75,000 copies, was an NPD Bookscan and Publisher’s Weekly Bestseller, broke the Top 40 on Amazon, and has more than 1,000 Five-star reviews. Mr. Handley and his nonspeaking son are also the authors of Underestimated: An Autism Miracle and co-produced the film SPELLERS, available now on YouTube.
How to End the Autism Epidemic is a reader-supported publication.
To receive new posts and support my work, consider becoming a free or paid subscriber.
-

 National1 day ago
National1 day agoCanada’s free speech record is cracking under pressure
-

 Energy21 hours ago
Energy21 hours agoTanker ban politics leading to a reckoning for B.C.
-

 Energy21 hours ago
Energy21 hours agoMeet REEF — the massive new export engine Canadians have never heard of
-

 Business1 day ago
Business1 day agoTaxpayers Federation calls on politicians to reject funding for new Ottawa Senators arena
-

 Censorship Industrial Complex2 days ago
Censorship Industrial Complex2 days agoOttawa’s New Hate Law Goes Too Far
-

 Fraser Institute22 hours ago
Fraser Institute22 hours agoClaims about ‘unmarked graves’ don’t withstand scrutiny
-

 Business21 hours ago
Business21 hours agoToo nice to fight, Canada’s vulnerability in the age of authoritarian coercion
-
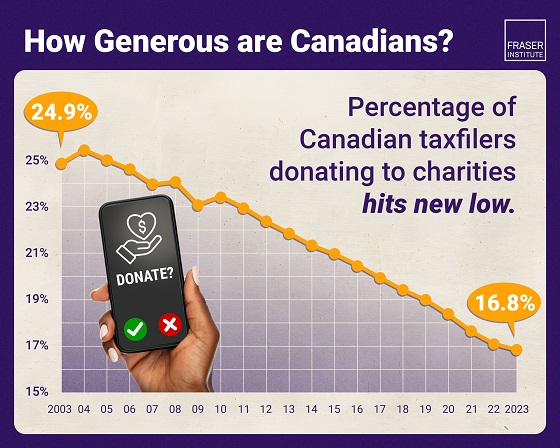
 Business1 day ago
Business1 day agoAlbertans give most on average but Canadian generosity hits lowest point in 20 years





