Frontier Centre for Public Policy
BC teacher fired for sharing the truth about Indian Residential Schools speaks out

From the Frontier Centre for Public Policy
By Jim McMurtry
The End of Nuance
George W. Bush said famously to Congress after the 9-11 terrorist attack, “Every nation in every region now has a decision to make. Either you are with us, or you are with the terrorists.” Similarly, George Orwell said in 1942 that “in practice, ‘he that is not with me is against me.’ The idea that you can somehow remain aloof from and superior to the struggle…is a bourgeois illusion.”
My employer, the Abbotsford School District, showed me that I was not aloof from the struggle by investigating me for “extremely serious misconduct.” I had relayed to senior history students the “most important news in Canada in 2021,” which was that the remains of 215 Indigenous children had been discovered in a mass grave in an apple orchard on the site of a former Indian residential school in Kamloops, British Columbia. This news led the Canadian government to declare itself guilty of genocide for having placed about one-third of First Nation children in long-ago-shuttered residential schools.
At the end of the partial and superficial investigation, I was fired. But, like the Joseph K. character in Franz Kafka’s The Trial, I knew I had done nothing wrong:
“But I’m not guilty,” said K. “there’s been a mistake. How is it even possible for someone to be guilty? We’re all human beings here, one like the other.” “That is true,” said the priest, “but that is how the guilty speak.”
My crime was in saying that most students who died while enrolled in these schools from 1883-1996 did so from disease, especially tuberculosis. Though factually true, the Abbotsford School District wrote to me in June 2021 that it was a time to hear from students “and not debate or challenge their emotional response to the news…. [Students] were struggling to make sense of the news and process the discovery.”
The problem was there was no discovery in Kamloops, and there still is no evidence whatsoever.
For the past three years the Royal Canadian Mounted Police have not conducted an investigation of the alleged murders; the archeologist’s report has been sealed by a local university (Simon Fraser University); and no excavation of the site has taken place.
The Abbotsford School District charged that “clearly it was not a time to play ‘devil’s advocate’ or to have a nuanced debate or discussion of the underlying reasons for these deaths…or push their thinking on the issue when students, as [with most] of the staff and general public, were struggling to make sense of the news.” It didn’t help that I was a history teacher: “Mr. McMurtry decided to use the class as an opportunity to teach a history lesson.”
The allegations kept changing. For example, they first accused me of saying “the deaths could not be called murder or cultural genocide,” but later they said that “my comments to students were inflammatory, inappropriate, insensitive and/or contrary to the school’s message of condolences and reconciliation.”
I noted, of course, that they replaced the word truth, as in Truth and Reconciliation, with condolences.
In time I realized that I was simply outside of an orthodoxy that was distorted by sensationalized headlines and cowardly journalistic practices. The woke employ a dual lens of good and bad, friend and foe. In their lynch-mob mentality, nuance and compassion get short shrift.
In the schools where I taught there is an administrative class of citizens who play the role of gatekeepers against unacceptable ideas, for they fear that a Trojan horse might get inside the walls and open the gate for other ideas to enter. In such a not-so-brave new world, you are with us or against us. There are no shades of allegiance, no nuance. As I was not with those administrators, they came for me.
I have been without a teaching job now for three years and my grievance against my employer is in abeyance while an investigation into my teaching by my regulatory body, the B.C. Teachers’ Regulation Branch (TRB), slowly unfolds. The TRB investigator’s report could take up to a year to write and a subsequent public hearing could consume another year of my life. The process is the punishment.
Jim McMurtry has taught in many subject areas in many places, including Switzerland where he was Principal of Neuchâtel Junior College. He lives in Surrey, B.C.
Business
Hudson’s Bay Bid Raises Red Flags Over Foreign Influence

From the Frontier Centre for Public Policy
A billionaire’s retail ambition might also serve Beijing’s global influence strategy. Canada must look beyond the storefront
When B.C. billionaire Weihong Liu publicly declared interest in acquiring Hudson’s Bay stores, it wasn’t just a retail story—it was a signal flare in an era where foreign investment increasingly doubles as geopolitical strategy.
The Hudson’s Bay Company, founded in 1670, remains an enduring symbol of Canadian heritage. While its commercial relevance has waned in recent years, its brand is deeply etched into the national identity. That’s precisely why any potential acquisition, particularly by an investor with strong ties to the People’s Republic of China (PRC), deserves thoughtful, measured scrutiny.
Liu, a prominent figure in Vancouver’s Chinese-Canadian business community, announced her interest in acquiring several Hudson’s Bay stores on Chinese social media platform Xiaohongshu (RedNote), expressing a desire to “make the Bay great again.” Though revitalizing a Canadian retail icon may seem commendable, the timing and context of this bid suggest a broader strategic positioning—one that aligns with the People’s Republic of China’s increasingly nuanced approach to economic diplomacy, especially in countries like Canada that sit at the crossroads of American and Chinese spheres of influence.
This fits a familiar pattern. In recent years, we’ve seen examples of Chinese corporate involvement in Canadian cultural and commercial institutions, such as Huawei’s past sponsorship of Hockey Night in Canada. Even as national security concerns were raised by allies and intelligence agencies, Huawei’s logo remained a visible presence during one of the country’s most cherished broadcasts. These engagements, though often framed as commercially justified, serve another purpose: to normalize Chinese brand and state-linked presence within the fabric of Canadian identity and daily life.
What we may be witnessing is part of a broader PRC strategy to deepen economic and cultural ties with Canada at a time when U.S.-China relations remain strained. As American tariffs on Canadian goods—particularly in aluminum, lumber and dairy—have tested cross-border loyalties, Beijing has positioned itself as an alternative economic partner. Investments into cultural and heritage-linked assets like Hudson’s Bay could be seen as a symbolic extension of this effort to draw Canada further into its orbit of influence, subtly decoupling the country from the gravitational pull of its traditional allies.
From my perspective, as a professional with experience in threat finance, economic subversion and political leveraging, this does not necessarily imply nefarious intent in each case. However, it does demand a conscious awareness of how soft power is exercised through commercial influence, particularly by state-aligned actors. As I continue my research in international business law, I see how investment vehicles, trade deals and brand acquisitions can function as instruments of foreign policy—tools for shaping narratives, building alliances and shifting influence over time.
Canada must neither overreact nor overlook these developments. Open markets and cultural exchange are vital to our prosperity and pluralism. But so too is the responsibility to preserve our sovereignty—not only in the physical sense, but in the cultural and institutional dimensions that shape our national identity.
Strategic investment review processes, cultural asset protections and greater transparency around foreign corporate ownership can help strike this balance. We should be cautious not to allow historically Canadian institutions to become conduits, however unintentionally, for geopolitical leverage.
In a world where power is increasingly exercised through influence rather than force, safeguarding our heritage means understanding who is buying—and why.
Scott McGregor is the managing partner and CEO of Close Hold Intelligence Consulting.
Business
Canada Urgently Needs A Watchdog For Government Waste

From the Frontier Centre for Public Policy
By Ian Madsen
From overstaffed departments to subsidy giveaways, Canadians are paying a high price for government excess
Not all the Trump administration’s policies are dubious. One is very good, in theory at least: the Department of Government Efficiency. While that term could be an oxymoron, like ‘political wisdom,’ if DOGE is useful, so may be a Canadian version.
DOGE aims to identify wasteful, duplicative, unnecessary or destructive government programs and replace outdated data systems. It also seeks to lower overall costs and ensure mechanisms are in place to evaluate proposed programs for effectiveness and value for money. This can, and usually does, involve eliminating some departments and, eventually, thousands of jobs. Some new roles within DOGE may need to become permanent.
The goal in the U.S. is to lower annual operating costs and ensure that the growth in government spending is lower than in revenues. Washington’s spending has exploded in recent years. The U.S. federal deficit exceeds six per cent of gross domestic product. According to the U.S. Treasury Department, annual debt service cost is escalating unsustainably.
Canada’s latest budget deficit of $61.9 billion in fiscal 2023–24 is about two per cent of GDP, which seems minor compared to our neighbour. However, it adds to the federal debt of $1.236 trillion, about 41 per cent of our approximate $3 trillion GDP. Ottawa’s public accounts show that expenses are 17.8 per cent of GDP, up from about 14 per cent just eight years ago. Interest on the escalating debt were 10.2 per cent of revenues in the most recent fiscal year, up from just five per cent a mere two years ago.
The Canadian Taxpayers Federation (CTF) continually identifies dubious or frivolous spending and outright waste or extravagance: “$30 billion in subsidies to multinational corporations like Honda, Volkswagen, Stellantis and Northvolt. Federal corporate subsidies totalled $11.2 billion in 2022 alone. Shutting down the federal government’s seven regional development agencies would save taxpayers an estimated $1.5 billion annually.”
The CTF also noted that Ottawa hired 108,000 more staff in the past eight years at an average annual cost of over $125,000. Hiring in line with population growth would have added only 35,500, saving about $9 billion annually. The scale of waste is staggering. Canada Post, the CBC and Via Rail lose, in total, over $5 billion a year. For reference, $1 billion would buy Toyota RAV4s for over 25,600 families.
Ottawa also duplicates provincial government functions, intruding on their constitutional authority. Shifting those programs to the provinces, in health, education, environment and welfare, could save many more billions of dollars per year. Bad infrastructure decisions lead to failures such as the $33.4 billion squandered on what should have been a relatively inexpensive expansion of the Trans Mountain pipeline—a case where hiring better staff could have saved money. Terrible federal IT systems, exemplified by the $4 billion Phoenix payroll horror, are another failure. The Green Slush Fund misallocated nearly $900 million.
Ominously, the fast-growing Old Age Supplement and Guaranteed Income Security programs are unfunded, unlike the Canada Pension Plan. Their costs are already roughly equal to the deficit and could become unsustainable.
Canada is sleepwalking toward financial perdition. A Canadian version of DOGE—Canada Accountability, Efficiency and Transparency Team, or CAETT—is vital. The Auditor General Office admirably identifies waste and bad performance, but is not proactive, nor does it have enforcement powers. There is currently no mechanism to evaluate or end unnecessary programs to ensure Canadians will have a prosperous and secure future. CAETT could fill that role.
Ian Madsen is the Senior Policy Analyst at the Frontier Centre for Public Policy.
-
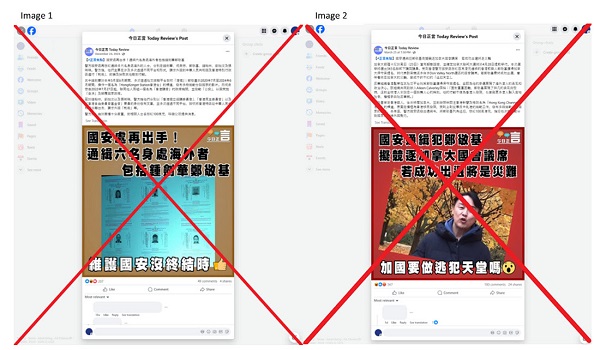
 2025 Federal Election1 day ago
2025 Federal Election1 day agoOttawa Confirms China interfering with 2025 federal election: Beijing Seeks to Block Joe Tay’s Election
-

 Energy2 days ago
Energy2 days agoIndigenous-led Projects Hold Key To Canada’s Energy Future
-

 Energy2 days ago
Energy2 days agoMany Canadians—and many Albertans—live in energy poverty
-

 Business2 days ago
Business2 days agoCanada Urgently Needs A Watchdog For Government Waste
-

 2025 Federal Election24 hours ago
2025 Federal Election24 hours agoHow Canada’s Mainstream Media Lost the Public Trust
-

 2025 Federal Election13 hours ago
2025 Federal Election13 hours agoBREAKING: THE FEDERAL BRIEF THAT SHOULD SINK CARNEY
-

 2025 Federal Election24 hours ago
2025 Federal Election24 hours agoReal Homes vs. Modular Shoeboxes: The Housing Battle Between Poilievre and Carney
-

 2025 Federal Election14 hours ago
2025 Federal Election14 hours agoCHINESE ELECTION THREAT WARNING: Conservative Candidate Joe Tay Paused Public Campaign





