MacDonald Laurier Institute
Are gender interventions helpful? First do no harm!: David Zitner for Inside Policy

From the Macdonald Laurier Institute
By David Zitner
Canadian public health bodies are lagging far behind peer nations in calling for evidenced-based approach to gender-affirming care.
Public health bodies in Europe and the United States are sounding the alarm, calling for serious research into the safety and benefits of youth gender transition interventions. On August 12, the American Society of Plastic Surgeons became the first major US medical association to challenge the consensus of medical groups over “gender-affirming care” for minors.
Meanwhile, systematic reviews of evidence conducted by public health authorities in Finland, Sweden, and the United Kingdom concluded that the risk/benefit ratio of youth gender transition ranges from unknown to unfavourable.
According to a US study, each stage of gender affirming treatment – social affirmation, pubertal blockade, administration of cross-sex hormones, and sex reassignment surgery – poses harms and risks that are not fully disclosed to minors and families.
Regulators in Europe and the UK have already reacted accordingly, with more emphasis on psychosocial support over medical interventions.
Canadian regulators, on the other hand, have been mum on the need for evidence-based treatment and they have not even insisted that proponents of aggressive treatments track the long-term physical and mental health outcomes of gender-affirming interventions.
Doctors and parents trying to support children with gender issues are faced with important, sometimes conflicting, moral and ethical dilemmas as they try to help distressed children while avoiding harm.
For most established treatments across the various areas of medicine, researchers have studied the chances and types of benefits and harms, the research is accessible, and patients can choose based on their own values and subjective assessment the anticipated benefits and risks. It is entirely different for those who consider gender-affirming interventions.
Parents acting as agents for children with gender identity issues are in a particularly desperate situation because of the absence of reliable research identifying those children who will someday detransition. Too many parents will likely feel deeply aggrieved when they realize that what might have been a passing fancy of childhood or adolescence produced permanent, irreversible harms.
In pursuing gender-affirming care, doctors and parents simply cannot avoid harm because the evidence is insufficient to identify those who might benefit from aggressive therapies and those who will be harmed.
Unfortunately, this absence of reliable scientific research does not prevent people, including Canadian regulators, from expressing strong opinions in favour of aggressive gender interventions.
Some adolescents experience gender fluidity and uncertainty as they grow. From time to time, a person might change how they feel, expressing their identity one way and subsequently another. Such persons might dress and accept the identity compatible with their genetic identity (male or female) at one period of their life, and at other times identify with or express an identity incompatible with their genetics. Like other emotional preferences, they can change over time.
A British Medical Journal article commenting on the Cass Review emphasized that anyone who genuinely cares for children should recognize that “gender medicine is built on shaky foundations.” Citing numerous articles in peer-reviewed pediatric journals, it concludes: “The evidence base for interventions in gender medicine is threadbare, whichever research question you wish to consider — from social transition to hormone treatment. Of more than one hundred studies examining the role of puberty blockers and hormone treatment for gender transition only two were of passable quality…. Intervention studies — particularly of drug and surgical interventions — should be designed to evaluate relevant outcomes with adequate follow-up.”
Despite the opinion of some professional gender-modification advocates, evidence of benefit is scant. On the other hand, the risk of aggressive gender-transition interventions include changes to fertility, bone, and cardiovascular health, and growth. It simply must be said that tampering with a young person’s hormone levels and appearance leads to dramatic, and often permanent, harmful biologic changes.
Canada’s peers are waking up to the dangers of gender-affirming care for minors and the threadbare evidence on which it has progressed thus far. Where is Canada?
Canadian public health bodies and regulators should follow other international bodies and insist on evidence-based care following the systematic study of the benefits and harms of aggressive gender interventions. Until that study is complete, we must insist that doctors remember their obligation to “do no harm.”
David Zitner is a senior fellow at the Macdonald-Laurier Institute. He has participated at every level of Canadian health care including clinical practice, research, administration, governance and patient and professional education.
Business
Closing information gaps to strengthen Canada’s border security and track fentanyl
By Sean Parker, Dawn Jutla, and Peter Copeland for Inside Policy
To promote better results, we lay out a collaborative approach
Despite exaggerated claims about how much fentanyl is trafficked across the border from Canada to the United States, the reality is that our detection, search, and seizure capacity is extremely limited.
We’re dealing with a “known unknown”: a risk we’re aware of, but don’t yet have the capacity to understand its extent.
What’s more, it may be that the flow of precursor chemicals—ingredients used in the production of fentanyl—is where much of the concern lies. Until we enhance our tracking, search, and seizure capacity, much will remain speculative.
As border security is further scrutinized, and the extent of fentanyl production and trafficking gets brought into sharper focus, the role of the federal government’s Precursor Chemical Risk Management Unit (PCRMU)—announced recently by Health Canada—will become apparent.
Ottawa recently took action to enhance the capabilities of the PCRMU. It says the new unit will “provide better insights into precursor chemicals, distribution channels, and enhanced monitoring and surveillance to enable timely law enforcement action.” The big question is, how will the PCRMU track the precursor drugs entering into Canada that are used to produce fentanyl?
Key players in the import-export ecosystem do not have the right regulatory framework and responsibilities to track and share information, detect suspect activities, and be incentivized to act on it. That’s one of the reasons why we know so little about how much fentanyl is produced and trafficked.
Without proper collaboration with industry, law enforcement, and financial institutions, these tracking efforts are doomed to fail. To promote better results, we lay out a collaborative approach that distributes responsibilities and retools incentives. These measures would enhance information collection capabilities, incentivize system actors to compliance, and better equip law enforcement and border security services for the safety of Canadians.
Trade-off bottleneck: addressing the costs of enhanced screening
To date, it’s been challenging to increase our ability to detect, search, and seize illegal goods trafficked through ports and border crossings. This is due to trade-offs between heightened manual search and seizure efforts at ports of entry, and the economic impacts of these efforts.
In 2024, the Canada Border Services Agency (CBSA) admitted over 93 million travelers. Meanwhile, 5.3 million trucks transported commercial goods into Canada, around 3.6 million shipments arrived via air cargo, nearly 2 million containers were processed at Canadian ports, roughly 1.9 million rail cars carried goods into the country, and about 145.7 million courier shipments crossed the border. The CBSA employs a risk-based approach to border security, utilizing intelligence, behavioral analysis, and random selection to identify individuals or shipments that may warrant additional scrutiny. This triaging process aims to balance effective enforcement with the facilitation of legitimate travel and trade.
Exact percentages of travelers subjected to secondary inspections are not publicly disclosed, but it’s understood that only a small fraction undergo such scrutiny. We don’t learn about the prevalence of these issues through our border screening measures, but in crime reporting data—after it’s too late to avert.
It’s key to have an approach that minimizes time and personnel resources deployed at points of entry. To be effective without being economically disruptive, policymakers, law enforcement, and border security need to strengthen requirements for information gathering, live tracking, and sharing. Legislative and regulatory change to require additional information of buyers and sellers—along with stringent penalties to enforce non-compliance—is a low-cost, logistically efficient way of distributing responsibility for this complex and multifaceted issue. A key concept explored in this paper is strengthening governance controls (“controls”) over fentanyl supply chains through new processes and data digitization, which could aid the PCRMU in their strategic objectives.
Enhanced supply chain controls are needed
When it comes to detailed supply chain knowledge of fentanyl precursor chemicals moving in and out of Canada, regulator knowledge is limited.
That’s why regulatory reform is the backbone of change. It’s necessary to ensure that strategic objectives are met by all accountable stakeholders to protect the supply chain and identify issues. To rectify the issues, solutions can be taken by the PCRMU to obtain and govern a modern fentanyl traceability system/platform (“platform”) that would provide live transparency to regulators.
A fresh set of supply chain controls, integrated into a platform as shown in Fig. 1, could significantly aid the PCRMU in identifying suspicious activities and prioritizing investigations.

Our described system has two distinctive streams: one which leverages a combination of physical controls such as package tampering and altered documentation against a second stream that looks at payment counterparties. Customs agencies, transporters, receivers, and financial institutions would have a hand in ensuring that controls in the platform are working. The platform includes several embedded controls to enhance supply chain oversight. It uses commercially available Vision AI to assess packaging and blockchain cryptography to verify shipment documentation integrity. Shipment weight and quantity are tracked from source to destination to detect diversion, while a four-eyes verification process ensures independent reconciliation by the seller, customs, and receiver. Additionally, payment details are linked to shipments to uncover suspicious financial activity and support investigations by financial institutions and regulators like FINTRAC and FINCEN.
A modern platform securely distributes responsibility in a way that’s cost effective and efficient so as not to overburden any one actor. It also ensures that companies of all sizes can participate, and protects them from exploitation by criminals and reputational damage.
In addition to these technological enhancements and more robust system controls, better collaboration between the key players in the fentanyl supply chain is needed, along with policy changes to incentivize each key fentanyl supply chain stakeholder to adopt the new controls.
Canadian financial institutions: a chance for further scrutiny
Financial institutions (FIs) are usually the first point of contact when a payment is being made by a purchaser to a supplier for precursor chemicals that could be used in the production of fentanyl. It is crucial that they enhance their screening and security processes.
Chemicals may be purchased by wires or via import letters of credit. The latter is the more likely of the two instruments to be used because this ensures that the terms and conditions in the letter of credit are met with proof of shipment prior to payment being released. Payments via wire require less transparency.
Where a buyer pays for precursor chemicals with a wire, it should result in further scrutiny by the financial institution. Requests for supporting documentation including terms and conditions, along with proof of shipment and receipt, should be provided. Under new regulatory policy, buyers would be required to place such supporting documentation on the shared platform.
The less transparent a payment channel is in relation to the supply chain, the more concerning it should be from a risk point of view. Certain payment channels may be leveraged to further mask illicit activity throughout the supply chain. At the onset of the relationship the seller and buyers would link payment information on the platform (payment channel, recipient name, recipient’s bank, date, and payment amount) to each precursor or fentanyl shipment. The supplier, in turn, should record match payment information (payment channel, supplier name, supplier’s bank, date, and payment amount).
Linking payment to physical shipment would enable data analytics to detect irregularities. An irregularity is flagged when the amounts and/or volume of payments far exceed the value of the received goods or vice versa. The system would be able to understand which fentanyl supply chains tend to use a particular set of FIs. This makes it possible to conduct real-time mapping of companies, their fentanyl and precursor shipments and receipts, and the payment institutions they use. With this bigger picture, FIs and law enforcement could connect the dots faster.
Live traceability reporting
Today, suppliers of fentanyl precursors are subject to the Pre-Export Notification Online (PEN Online) database. This database enables governments to monitor international trade in precursor chemicals by sending and receiving pre-export notifications. The system helps prevent the diversion of chemicals used in the illicit manufacture of drugs by allowing authorities to verify the legitimacy of shipments before they occur.
To further strengthen oversight, the platform utilizes immutability technologies—such as blockchain or secure immutable databases—which can be employed to encrypt all shipping documents and securely share them. This presents an auditable form of chain-of-custody and makes any alterations apparent. Customs and buyers would have the capability to verify the authenticity of the originating documents in a way that doesn’t compromise business confidentiality. With the use of these technologies, law enforcement can narrow down their investigations.
An information gap currently exists as the receivers of the shipments don’t share their receipts information with PEN. To strengthen governance on fentanyl supply chains, regulatory policy and legislative changes are needed. The private sector should be mandated to report received quantities of fentanyl or its precursors, as well as suspicious receiving destinations. This could be accomplished on the platform which would embed the receiving process, a reconciliation process of the transaction, the secure upload and sharing of documents, and would be minimally disruptive to business processes.
Additionally, geo-location technology embedded in mobile devices and/or shipments would provide real-time location-based tracking of custody transactions. These geo-controls would ensure accountability across the fentanyl supply chain, in particular where shipments veer off or stop too long on regular shipping routes. Canadian transporters of fentanyl and its precursor chemicals should play an important role in detecting illicit diversion/activities.
Digital labelling
Licensed fentanyl manufacturers could add new unique digital labels to their shipments to get expedited clearance. For example, immutable digital labelling platforms enable tamper-proof digital labels for legitimate fentanyl shipments. This would give pharmacies, doctors, and regulators transparency into the fentanyl’s:
- Chemical composition and concentrations (determining legitimate vs. adulterated versions of the drug)
- Manufacturing facility ID, batch ID, and regulatory compliance status
- Intended buyer authentication (such as licensed pharmaceutical firms or distributors)
Immutable digital labelling platforms offer secure role-based access control. They can display customized data views according to time of day, language, and location. Digital labels could enable international border agencies and law enforcement to receive usable data, allowing legal shipments through faster while triggering closer shipment examinations for those without of a digital label.
International and domestic transporter controls
Transporters act as intermediaries in the supply chain. Their operations could be monitored through a regulatory policy that mandates their participation in the platform for fentanyl and precursor shipments. The platform would support a mobile app interface for participants on-the-move, as well as a web portal and application programming interfaces (APIs) for large-size supply chain participants. Secure scanning of packaging at multiple checkpoints, combined with real-time tracking, would provide an additional layer of protection against fraud, truckers taking bribes, and unauthorized alterations to shipments and documents.
Regulators and law enforcement participation
Technology-based fentanyl controls for suppliers, buyers, and transporters may be reinforced by international customs and law enforcement collaboration on the platform. Both CBSA and law enforcement could log in and view alerts about suspicious activities issued from the FIs, transporters, or receivers. The reporting would allow government personnel to view a breakdown of fentanyl importers, the number of import permit applications, and the amount of fentanyl and its precursors flowing into the country. Responsible regulatory agencies—such as the CBSA and PCRMU—could leverage the reporting to identify hot spots.
The platform would use machine learning to support CBSA personnel in processing an incoming fentanyl or precursor shipment. Machine learning refers to AI algorithms and systems that improve their knowledge with experience. For example, an AI assistant on the traceability system could use machine learning to predict and communicate which import shipments arriving at the border should be passed. It can base these suggestions on criteria like volume, price, origin of raw materials, and origin of material at import point. It can also leverage data from other sources such as buyers, sellers, and banks to make predictions. As an outcome, the shipment may be recommended to pass, flagged as suspicious, or deemed to require an investigation by CBSA.
It’s necessary to keep up to date on new precursor chemicals as the drug is reformulated. Here, Health Canada can play a role, using its new labs and tests—expected as part of the recently announced Canadian Drug Analysis Centre—to provide chemical analysis of seized fentanyl. This would inform which additional chemical supply chains should be tracked in the PCRMU’s collaborative platform, and all stakeholders would widen their scope of review.
These new tools would complement existing cross-border initiatives, including joint U.S.-Canada and U.S.-Mexico crackdowns on illicit drug labs, as well as sovereign efforts. They have the potential to play a vital role in addressing fentanyl trafficking.
A robust, multi-pronged strategy—integrating existing safeguards with a new PCRMU traceability platform—could significantly disrupt the illegal production and distribution of fentanyl. By tracking critical supply chain events and authenticating shipment data, the platform would equip law enforcement and border agencies in Canada, the U.S., and Mexico with timely, actionable intelligence. The human toll demands urgency: from 2017 to 2022, the U.S. averaged 80,000 opioid-related deaths annually, while Canada saw roughly 5,500 per year from 2016 to 2024. In just the first nine months of 2024, Canadian emergency services responded to 28,813 opioid-related overdoses.
Combating this crisis requires more than enforcement. It demands enforceable transparency. Strengthened governance—powered by advanced traceability technology and coordinated public-private collaboration—is essential. This paper outlines key digital controls that can be implemented by global suppliers, Canadian buyers, transporters, customs, and financial institutions. With federal leadership, Canada can spearhead the adoption of proven, homegrown technologies to secure fentanyl supply chains and save lives.
Sean Parker is a compliance leader with well over a decade of experience in financial crime compliance, and a contributor to the Macdonald-Laurier Institute.
Dawn Jutla is the CEO of Peer Ledger, the maker of a traceability platform that embeds new control processes on supply chains, and a professor at the Sobey School of Business.
Peter Copeland is deputy director of domestic policy at the Macdonald-Laurier Institute.
MacDonald Laurier Institute
Rushing to death in Canada’s MAiD regime

 By Ramona Coelho for Inside Policy
By Ramona Coelho for Inside Policy
Canada legalized Medical Assistance in Dying (MAiD) in 2016, encompassing both euthanasia and assisted suicide. Initially limited to those nearing their natural death, eligibility expanded in 2021 to individuals with physical disabilities, with eligibility for individuals with mental illness in 2027. Parliamentary recommendations include MAiD for children. A recent federal consultation explored extending MAiD to those who lack capacity via advance directives, an approach Quebec has already adopted, despite its criminal status under federal law.
Despite its compassionate framing, investigative journalists and government reports reveal troubling patterns where inadequate exploration of reversible suffering – such as lack of access to medical treatments, poverty, loneliness, and feelings of being a burden – have driven Canadians to choose death. As described by our former Disability Inclusion Minister, Canada’s system at times makes it easier to access MAiD than to receive basic care like a wheelchair. With over 60,000 MAiD cases by the end of 2023, the evidence raises grave concerns about Canada’s MAiD regime.
I am a member of Ontario’s MAiD Death Review Committee (MDRC). Last year, the Chief Coroner released MDRC reports, and a new set of reports has just been published. The first report released by the Office of the Chief Coroner, Waivers of Final Consent, examines how individuals in Track 1 (reasonably foreseeable natural death) can sign waivers to have their lives ended even if they lose the capacity to consent by the scheduled date of MAiD. The second, Navigating Complex Issues within Same Day and Next Day MAiD Provisions, includes cases where MAiD was provided on the same day or the day after it was requested. These reports raise questions about whether proper assessments, thorough exploration of suffering, and informed consent were consistently practised by MAiD clinicians. While MDRC members hold diverse views, here is my take.
Rushing to death, Ignoring Reversible Causes of Suffering
In the same-day or next-day MAiD report, Mrs. B, in her 80s, after complications from surgery, opted for palliative care, leading to discharge home. She later requested a MAiD assessment, but her assessor noted she preferred palliative care based on personal and religious values. The next day, her spouse, struggling with caregiver burnout, took her to the emergency department, but she was discharged home. When a request for hospice palliative care was denied, her spouse contacted the provincial MAiD coordination service for an urgent assessment. A new assessor deemed her eligible for MAiD, despite concerns from the first practitioner, who questioned the new assessor on the urgency, the sudden shift in patient perspective, and the influence of caregiver burnout. The initial assessor requested an opportunity for re-evaluation, but this was denied, with the second assessor deeming it urgent. That evening, a third MAiD practitioner was brought in, and Mrs. B underwent MAiD that night.
The focus should have been on ensuring adequate palliative care and support for Mrs. B and her spouse. Hospice and palliative care teams should have been urgently re-engaged, given the severity of the situation. Additionally, the MAiD provider expedited the process despite the first assessor’s and Mrs. B’s concerns without fully considering the impact of her spouse’s burnout.
The report also has worrying trends suggesting that local medical cultures—rather than patient choice—could be influencing rushed MAiD. Geographic clustering, particularly in Western Ontario, where same-day and next-day MAiD deaths occur most frequently, raises concerns that some MAiD providers may be predisposed to rapidly approve patients for quick death rather than ensuring patients have access to adequate care or exploring if suffering is remediable. This highlights a worrying trend where the speed of the MAiD provision is prioritized over patient-centered care and ethical safeguards.
MAiD without Free and Informed Choice
Consent has been central to Canadians’ acceptance of the legalization of euthanasia and assisted suicide. However, some cases in these reports point to concerns already raised by clinicians: the lack of thorough capacity assessments and concerns that individuals may not have freely chosen MAiD.
In the waiver of final consent report, Mr. B, a man with Alzheimer’s, had been approved for MAiD with such a waiver. However, by the scheduled provision date, his spouse reported increased confusion. Upon arrival, the MAiD provider noted that Mr. B no longer recognized them and so chose not to engage him in discussion at all. Without any verbal interaction to determine his current wishes or understanding, Mr. B’s life was ended.
In the same-day or next-day MAiD report, Mr. C, diagnosed with metastatic cancer, initially expressed interest in MAiD but then experienced cognitive decline and became delirious. He was sedated for pain management. Despite the treating team confirming that capacity was no longer present, a MAiD practitioner arrived and withheld sedation, attempting to rouse him. It was documented that the patient mouthed “yes” and nodded and blinked in response to questions. Based on this interaction, the MAiD provider deemed the patient to have capacity. The MAiD practitioner then facilitated a virtual second assessment, and MAiD was administered.
These individuals were not given genuine opportunities to confirm whether they wished to die. Instead, their past wishes or inquiries were prioritized, raising concerns about ensuring free and informed consent for MAiD. As early as 2020, the Chief Coroner of Ontario identified cases where patients received MAiD without well-documented capacity assessments, even though their medical records suggested they lacked capacity. Further, when Dr. Leonie Herx, past president of the Canadian Society of Palliative Medicine, testified before Parliament about MAiD frequently occurring without capacity, an MP dismissed her, advising Parliament to be cautious about considering seriously evidence under parliamentary immunities that amounted to malpractice allegations, which should be handled by the appropriate regulatory bodies or police. These dismissive comments stand in stark contrast with the gravity of assessing financial capacity, and yet the magnitude is greater when ending life. By way of comparison, for my father, an Ontario-approved capacity expert conducted a rigorous evaluation before declaring him incapable of managing his finances. This included a lengthy interview, collateral history, and review of financial documents—yet no such rigorous capacity assessment is mandated for MAiD.
What is Compassion?
While the federal government has finished its consultation on advance directives for MAiD, experts warn against overlooking the complexities of choosing death based on hypothetical suffering and no lived experience to inform those choices. A substitute decision-maker has to interpret prior wishes, leading to guesswork and ethical dilemmas. These cases highlight how vulnerable individuals, having lost the capacity to consent, may be coerced or unduly influenced to die—whether through financial abuse, caregiver burnout, or other pressures—reminding us that the stakes are high – life and death, no less.
The fundamental expectation of health care should be to rush to care for the patient, providing support through a system that embraces them—not rush them toward death without efforts to mitigate suffering or ensure free and informed consent. If we truly value dignity, we must invest in comprehensive care to prevent patients from being administered speedy death in their most vulnerable moment, turning their worst day into potentially their last.
Dr. Ramona Coelho is a family physician whose practice largely serves marginalised persons in London, Ontario. She is a senior fellow at the Macdonald-Laurier Institute and co-editor of the new book “Unravelling MAiD in Canada” from McGill University Press.
-
 2025 Federal Election2 days ago
2025 Federal Election2 days agoOttawa Confirms China interfering with 2025 federal election: Beijing Seeks to Block Joe Tay’s Election
-

 2025 Federal Election1 day ago
2025 Federal Election1 day agoBREAKING: THE FEDERAL BRIEF THAT SHOULD SINK CARNEY
-

 2025 Federal Election2 days ago
2025 Federal Election2 days agoHow Canada’s Mainstream Media Lost the Public Trust
-

 2025 Federal Election2 days ago
2025 Federal Election2 days agoReal Homes vs. Modular Shoeboxes: The Housing Battle Between Poilievre and Carney
-

 2025 Federal Election1 day ago
2025 Federal Election1 day agoCHINESE ELECTION THREAT WARNING: Conservative Candidate Joe Tay Paused Public Campaign
-

 Media1 day ago
Media1 day agoCBC retracts false claims about residential schools after accusing Rebel News of ‘misinformation’
-
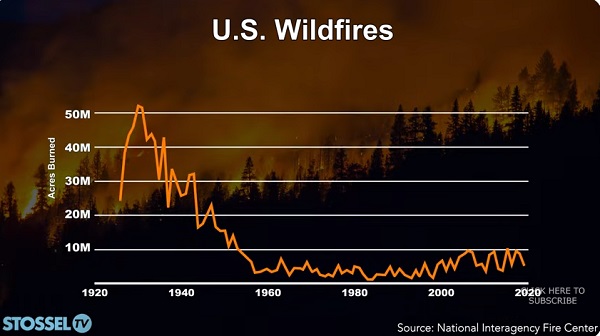
 John Stossel1 day ago
John Stossel1 day agoClimate Change Myths Part 2: Wildfires, Drought, Rising Sea Level, and Coral Reefs
-

 COVID-192 days ago
COVID-192 days agoNearly Half of “COVID-19 Deaths” Were Not Due to COVID-19 – Scientific Reports Journal





